A review of the totality of evidence in the development of ABP 798, a rituximab biosimilar
Abstract
ABP 798 (RIABNI™) is a biosimilar to rituximab reference product (RP), a monoclonal antibody that targets CD20. Approval of ABP 798 was based on the totality of evidence generated using a stepwise approach which began by showing that it is structurally and functionally similar to rituximab RP. This analytical assessment was followed by a demonstration of pharmacokinetic/pharmacodynamic similarity in patients with rheumatoid arthritis. Comparative clinical efficacy and safety of ABP 798 with rituximab RP was demonstrated as a final step in patients with non-Hodgkin lymphoma and in those with rheumatoid arthritis. Overall, the totality of evidence supported the conclusion that ABP 798 is highly similar to rituximab RP and provided scientific justification for extrapolation to other approved indications of rituximab RP.
ABP 798‡ (RIABNI™; rituximab-arrx; Amgen, Inc., CA, USA) is a biosimilar to Rituxan® (rituximab reference product [RP]; marketed as MabThera® outside the USA, Japan and Canada), a chimeric mouse/human monoclonal IgG1κ antibody that binds to CD20 expressed on the surface of mature B cells and most malignant B cells [1–3]. Upon binding to CD20, rituximab RP mediates B-cell lysis by several mechanisms, including complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) [1,4,5]. Because rituximab RP mediates its therapeutic effect by targeted depletion of B cells, it is used to treat oncological and autoimmune diseases in which B cells are excessive in number, overactive or dysfunctional. Specifically, rituximab RP is approved for indolent and aggressive forms of B-cell non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia, granulomatosis with polyangiitis and microscopic polyangiitis, moderate-to-severe pemphigus vulgaris and moderate-to-severe rheumatoid arthritis (RA) [2]. ABP 798 is currently approved in the USA and Canada for the same indications as rituximab RP, except for pemphigus vulgaris and RA in the USA [2,3,6].
Biosimilars are biological medicines that are highly similar to a licensed biological product in structure, purity, pharmacokinetics (PK), pharmacodynamics (PD), mechanism of action (MOA), potency, efficacy, safety and immunogenicity [7,8]. More specifically, developers must demonstrate that a biosimilar has no clinically meaningful differences from the RP to obtain regulatory approval. To spur competition in the biologics space, the US FDA created an abbreviated yet robust regulatory and approval pathway for biosimilars [9,10]. Given that biologics are produced in cell lines using complex manufacturing processes, a biosimilar, unlike a small-molecule generic drug, is not an exact copy of the RP [11–13]. Although biosimilars have highly similar structural and functional attributes to an approved biologic, they may have minor differences in clinically inactive components [8,9,14].
The biosimilar development process is based on guidance from the FDA and EMA and focuses on building a totality of evidence (TOE) which aims to demonstrate a high degree of similarity between the biosimilar candidate and the RP, forming the basis for its regulatory approval [15,16]. This review highlights the TOE for ABP 798 which supports its similarity to rituximab RP and regulatory approval.
Development of ABP 798
The development of ABP 798 utilized a step-by-step approach which began with a comparative analytical assessment using state-of-the-art techniques to evaluate its structural and functional attributes. Analytical assessment was followed by PK/PD studies in humans, and then two comparative clinical trials to confirm similar efficacy, safety and immunogenicity [8–10,15,17–22].
Comparative analytical & functional similarity assessment
Building of the ABP 798 TOE occurred in several stages, beginning with an extensive series of comparative structural analyses and functional assays to understand the critical quality attributes (CQAs) of rituximab RP [18]. CQAs are features (e.g., primary structure, posttranslational modifications, stability, purity and biological activity) that can impact safety, potency, PK and quality [9,11,23]. CQAs must closely match those of the RP to avoid clinically meaningful differences. A cell line producing ABP 798 was selected and manufacturing processes were developed [18]. A thorough analytical assessment was conducted to evaluate the structural and functional similarity of ABP 798 and rituximab RP, and to determine whether any differences had the potential to impact product efficacy or safety [18]. As recommended by regulatory authorities, ABP 798 and rituximab RP sourced from the USA and EU were assessed in three pairwise analytical comparisons of biological activity, primary structure, higher-order structure, particles and aggregates, product-related substances and impurities, thermal stability and forced degradation, and general properties. Evaluation of both rituximab US and rituximab EU facilitated the development of a scientific bridge such that a single RP can be used in clinical studies and supports approval in countries where the local RPs were not used in the clinical studies.
Multiple complementary methods, including reduced peptide map analysis, confirmed that ABP 798, rituximab RP US and rituximab RP EU have the same amino acid sequence and disulfide linkage patterns, and share common characteristics in their primary structures [18]. In addition, Fourier transform infrared spectroscopy, ultraviolet circular dichroism spectroscopy and differential scanning calorimetry were employed to demonstrate that ABP 798 and rituximab RP have similar secondary and tertiary structures. The N-linked glycosylation in ABP 798 and rituximab RP was confirmed by glycan map. The overlay of glycan maps demonstrated that ABP 798 and rituximab RP have similar glycan profiles, and that all major glycans are present in both ABP 798 and rituximab RP. The results also demonstrated that ABP 798 and rituximab RP have similar levels of high mannose, afucosylation, galactosylation and sialylation [18]. The physicochemical properties of size and charge variants were assessed using a combination of methods. ABP 798 has similar levels of main peak and size variants compared with rituximab RP as evaluated by size-exclusion HPLC and reduced and non-reduced capillary electrophoresis-sodium dodecyl sulfate. ABP 798 also has similar levels of charge variants compared with rituximab RP, as measured by cation exchange chromatography. Minor differences in biochemical attributes irrelevant to the MOA of rituximab RP were not considered to be clinically meaningful.
Functional assessment of ABP 798 included non-clinical evaluation of crucial biological activities that involve its antigen-binding (Fab) and complement and Fcγ receptor-binding (Fc) regions. The clinical efficacy of ABP 798 and rituximab RP are primarily mediated through the induction of effector functions, including CDC, ADCC, antibody-dependent cell-mediated phagocytosis (ADCP) and direct apoptosis (Figure 1) [1,4,5,24]. The binding of rituximab RP to CD20 triggers the activation of the C1q cascade that leads to deposition of complement molecules on the B-cell surface and apoptosis by CDC [4,25]. ADCC also causes B-cell depletion when rituximab RP binds to CD20 and to Fcγ receptors expressed on phagocytic cells, which then release immune mediators that kill target cells [1,4,25]. Moreover, ADCP may also play a key role in the destruction of B cells. Rituximab RP-opsonized B cells activate Fcγ receptors on the surface of macrophages to induce phagocytosis and degradation [26]. The cross-linking of rituximab RP bound to CD20 induces apoptosis through a signaling pathway that involves Src kinase activation [1].
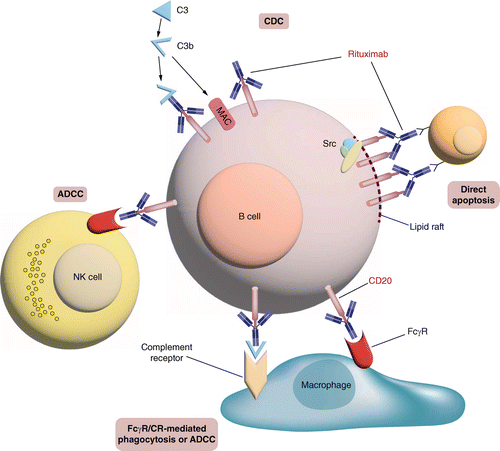
Rituximab-coated B cells are killed by at least four different mechanisms. Binding of rituximab to CD20 on the B-cell surface causes activation of the complement cascade, which generates the membrane attack complex that can directly induce B-cell lysis by complement-dependent cytotoxicity. Binding of rituximab allows interaction with NK cells via Fc receptors III (FcRIII), which leads to antibody-dependent cell-mediated cytotoxicity. The Fc portion of rituximab and the deposited complement fragments allow for recognition by both FcR and complement receptors on macrophages, which lead to antibody-dependent cell-mediated phagocytosis and antibody-dependent cell-mediated cytotoxicity. The crosslinking of several molecules of rituximab and CD20 in the lipid raft determines the interaction of these complexes with elements of a signaling pathway involving Src kinases that mediate direct apoptosis.
ADCC: Antibody-dependent cell-mediated cytotoxicity; ADCP: Antibody-dependent cell-mediated phagocytosis; CDC: Complement-dependent cytotoxicity; MAC: Membrane attack complex.
Reproduced with permission from [24], © Elsevier (2010) on behalf of The American Society of Hematology.
As evidenced by results from cell-based assays and binding studies, ABP 798, rituximab US and rituximab EU were similar in their biological and functional properties, including ADCC, CDC and ADCP activities (Figure 2) [18]. Although the apoptosis assay results show that ABP 798 has a marginally lower mean value versus rituximab RP, this difference is not regarded as clinically meaningful. Additional non-clinical assessments found that ABP 798 and rituximab RP are comparable in terms of CD20 binding, internalization of CD20, Fcγ receptor binding in primary cells, in vitro synergy with chemotherapeutic agents, in vivo efficacy in mouse xenograft models, and pharmacological B-cell depletion and toxicokinetics in cynomolgus monkeys [18,20].

(A) NK92 ADCC activity. (B) CDC potency. (C) Apoptosis activity. (D) ADCP activity.
ADCC: Antibody-dependent cell-mediated cytotoxicity; ADCP: Antibody-dependent cell-mediated phagocytosis; CDC: Complement-dependent cytotoxicity.
Reproduced with permission from [18], © Elsevier (2021) on behalf of the International Alliance for Biological Standardization.
Taken together, these non-clinical results suggest that ABP 798 is highly similar to rituximab RP. In addition, the similarity of ABP 798 to both rituximab RP US and rituximab RP EU established a scientific bridge that allowed a single RP to be used in clinical studies and supports approval in other countries where the local RPs were not used in the trials [9,17]. With the confirmation of similarity in structural and functional attributes, the development of ABP 798 proceeded to an evaluation of its PK/PD profile.
PK/PD Assessment
As the next step in biosimilar development, human PK/PD assessments are important to compare drug exposure, safety and immunogenicity of the candidate biosimilar and the RP.
The PK/PD similarity of ABP 798 with rituximab RP was demonstrated in patients with RA [21]. These pharmacological assessments were not conducted in healthy volunteers due to the potential health risks associated with B-cell depletion [27]. Patients were diagnosed based on the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria [28]. A total of 311 subjects aged 18–80 years with active RA were randomized and treated with two doses of ABP 798, rituximab RP US or rituximab RP EU, each consisting of two 1000-mg infusions 2 weeks apart. The PK end points included area under the serum concentration–time curve from time 0 to infinity (AUCinf) and the maximum observed drug concentration after the second infusion of the first dose (Cmax2). PD was evaluated by measuring the percentage of subjects with complete depletion in CD19+ cell count on day 3. Because rituximab RP and ABP 798 block available CD20 binding sites, the antibody used for flow cytometric assays cannot recognize the CD20 molecule on B cells [29]; thus CD19, another differentiation marker expressed on B cells, was used to measure the extent of B-cell depletion with rituximab RP and ABP 798. As shown in Figure 3, the mean concentration–time profiles were highly similar among ABP 798, rituximab RP US and rituximab RP EU. The PK similarity demonstration was based on the 90% CIs for AUCinf and Cmax2 falling within a prespecified margin of 0.8 and 1.25 for overall exposure, the standard for demonstrating PK bioequivalence. ABP 798, rituximab RP US and rituximab RP EU were also similar with respect to secondary PK end points, including AUC0–14 days, AUC0–12 wks, Cmax1, terminal elimination half-life and clearance [21]. In addition, the three study groups had similar PD effects, as demonstrated by the percentage of subjects showing complete CD19+ B-cell depletion from day 1 to day 3.

Reproduced with permission from [21] under the terms of the Creative Commons CC BY license.
The results indicate that ABP 798 has PK/PD similar to rituximab RP in RA patients. Given that PK/PD studies are a critical component in establishing biosimilarity, these results supported the further clinical development of ABP 798 as a proposed biosimilar to rituximab RP. It is worth noting that the results of a PK/PD assessment in NHL patients are provided below.
Comparative clinical efficacy & safety
Demonstration of equivalent clinical safety and efficacy was the final step in building the TOE needed to support the approval of ABP 798. Comparative clinical trials were conducted in two indications of rituximab RP: RA and NHL. The treatment effect of rituximab RP has been shown to be robust in prior trials of these two homogeneous and sensitive study populations, which thus enhanced the ability to detect any clinically meaningful differences between ABP 798 and rituximab RP.
Comparative clinical efficacy & safety in RA
Numerous clinical trials have established rituximab RP as efficacious and safe in RA [30–35]. The clinical similarity of ABP 798 and rituximab RP in patients with moderate-to-severe RA was demonstrated in a randomized, double-blind, active-controlled comparative clinical study conducted at 57 centers in Europe and the USA [22]. Study subjects received two 1000-mg intravenous infusions of ABP 798, rituximab RP US or rituximab RP EU, administered 2 weeks apart. At week 24 the ABP 798 and rituximab RP EU groups received the second dose of the same treatment, while those in the rituximab RP US group received ABP 798. The primary efficacy end point was the change from baseline of Disease Activity Score-28 for Rheumatoid Arthritis with CRP (DAS28-CRP), a measure of the severity of disease which combines information from swollen joints, tender joints, acute-phase response and general health [36]. DAS28-CRP is a continuous variable that is suitable for comparing two similar medications [37,38]. The safety and immunogenicity profiles of ABP 798 were also assessed.
As shown in Figure 4, the mean decrease from baseline in the primary efficacy end point, DAS28-CRP, was -2.197 (standard deviation: 1.3689) for ABP 798 and -2.125 (standard deviation: 1.3250) for rituximab RP at week 24 [22]. These scores were comparable with those observed in pivotal trials of the RP [31,33]. Per protocol specification, the rituximab RP US and rituximab RP EU groups were pooled into a single reference group for the primary assessment of clinical equivalence. The 90% CI (-0.225 to 0.264) for the mean difference at week 24 between the groups was within the predefined equivalence margin (-0.6 to 0.6). These data indicate that ABP 798 and rituximab RP are clinically equivalent. The results from secondary end points demonstrated comparable efficacy across treatment groups throughout the study and were also indicative of clinical similarity. For example, mean decreases from baseline in DAS28-CRP were similar in the ABP 798/ABP 798, rituximab RP EU/rituximab RP EU and rituximab RP US/ABP 798 groups up to week 48, demonstrating that improvement in disease severity was sustained through the study period. In addition, the American College of Rheumatology (ACR) ACR20, ACR50 and ACR70 responses among the groups were also similar [22].

RP: Reference product.
Reproduced with permission from [22] under the terms of the Creative Commons CC BY license.
In terms of safety, the frequency, type and severity of adverse events (AEs) were comparable (Table 1) [22] and within the range previously described for rituximab RP [2,31,33]. During the study [22], grade ≥3 AEs were experienced by five (4.8%), nine (8.7%) and nine (8.7%) subjects in the ABP 798/ABP 798, rituximab RP EU/rituximab RP EU and rituximab RP US/ABP 798 groups, respectively. Serious AEs (SAEs) were similar across the groups. AEs resulting in drug or study discontinuation occurred in 12 subjects (ABP 798/ABP 798: three [2.9%]; rituximab RP EU/rituximab RP EU: two [1.9%]; rituximab RP US/ABP 798: seven [6.8%]), with infusion reactions being the most common reason for discontinuation. In addition, subjects who underwent a single switch from rituximab RP US to ABP 798 had similar levels of binding and neutralizing anti-drug antibodies (ADAs) as well as all grade AEs, grade ≥3 AEs, SAEs, AEs of interest and AEs leading to drug or study discontinuations [22].
| ABP 798/ABP 798 (n = 104) | Rituximab (EU)/Rituximab (EU) (n = 104) | Rituximab (US)/ABP 798 (n = 103) | |
|---|---|---|---|
| Day 1 until first or second infusion | |||
| Any adverse event, n (%) | 52 (50.0) | 44 (42.3) | 44 (42.7) |
| Any ≥grade 3 adverse event, n (%) | 4 (3.8) | 6 (5.8) | 4 (3.9) |
| Any fatal adverse event, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any serious adverse event, n (%) | 4 (3.8) | 5 (4.8) | 5 (4.9) |
| Any adverse event leading to discontinuation of IP/study, n (%) | 3 (2.9) | 1 (1.0) | 4 (3.9) |
| Adverse events of interest, n (%) | 19 (18.3) | 11 (10.6) | 18 (17.5) |
| Infusion reactions including hypersensitivity | 12 (11.5) | 7 (6.7) | 12 (11.7) |
| Hematological reactions | 4 (3.8) | 2 (1.9) | 3 (2.9) |
| Serious infections | 2 (1.9) | 3 (2.9) | 1 (1.0) |
| Cardiac disorders | 2 (1.9) | 2 (1.9) | 2 (1.9) |
| Opportunistic infection | 1 (1.0) | 0 (0.0) | 1 (1.0) |
| Day 1 through end of study | |||
| Any adverse event, n (%) | 67 (64.4) | 54 (51.9) | 56 (54.4) |
| Any ≥grade 3 adverse event, n (%) | 5 (4.8) | 9 (8.7) | 9 (8.7) |
| Any fatal adverse event, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any serious adverse event, n (%) | 8 (7.7) | 8 (7.7) | 8 (7.8) |
| Any adverse event leading to discontinuation of IP/study, n (%) | 3 (2.9) | 2 (1.9) | 7 (6.8) |
| Adverse events of interest, n (%) | 25 (24.0) | 15 (14.4) | 23 (22.3) |
| Infusion reactions including hypersensitivity | 16 (15.4) | 9 (8.7) | 16 (15.5) |
| Hematological reactions | 5 (4.8) | 2 (1.9) | 7 (6.8) |
| Serious infections | 4 (3.8) | 4 (3.8) | 1 (1.0) |
| Cardiac disorders | 4 (3.8) | 3 (2.9) | 2 (1.9) |
| Opportunistic infection | 1 (1.0) | 2 (1.9) | 1 (1.0) |
Comparative clinical efficacy, safety & PK/PD in NHL
Rituximab RP, both as monotherapy and in combination with chemotherapy, has been demonstrated to significantly delay disease progression and death, with limited side effects, and has changed the paradigm for treating NHL [39–41]. The clinical similarity of ABP 798 and rituximab RP in patients with NHL was examined in the JASMINE study [19]. This randomized, double-blind, comparative clinical trial was designed to evaluate the efficacy, PK/PD, safety and immunogenicity of ABP 798 compared with rituximab RP in subjects ≥18 years of age with previously untreated CD20+, low-tumor-burden follicular lymphoma. Follicular lymphoma, a subtype of B-cell NHL characterized as indolent in nature [42], is the second most common lymphoma diagnosed in the USA and western Europe. The choice of follicular lymphoma patients allowed for study in a homogeneous population. A total of 256 patients were randomized to receive a 375 mg/m2 intravenous infusion of either ABP 798 or rituximab RP once weekly for 4 weeks, followed by dosing at weeks 12 and 20 [19]. The primary end point was a central, independent, blinded assessment of the risk difference (RD) of overall response rate (ORR) by Week 28. ORR, a sensitive end point that provides a direct measure of antitumor activity and that can be assessed within a short time frame, is adequate for detecting potential product-related differences in a comparative clinical study. Secondary end points included RD of ORR at week 12, AEs and the incidence of ADAs.
Overall, there were no clinically meaningful differences in efficacy or safety of ABP 798 compared with rituximab RP in patients with follicular lymphoma [19]. As shown in Table 2, 96 patients (78.0%) in the ABP 798 group and 87 patients (70.2%) in the rituximab RP group had a best ORR by week 28. The one-sided 95% lower confidence limit of RD of ORR by week 28 was -1.4% and was within the prespecified noninferiority margin of -15%. Additionally, the one-sided 95% upper confidence limit was determined to be 16.8% and was within the prespecified nonsuperiority margin of 35.5%. The results of the sequential testing thus established equivalence in clinical efficacy between ABP 798 and rituximab RP. Moreover, 73 patients (59.3%) and 72 patients (58.1%) in the ABP 798 and rituximab RP groups, respectively, had best overall response at week 12, supporting a conclusion of clinical similarity.
| ABP 798 (n = 123) | Rituximab RP (n = 124) | |
|---|---|---|
| ORR†, n (%) | 96 (78.0) | 87 (70.2) |
| 95% CI of ORR (%) | (70.7–85.4) | (62.1–78.2) |
| RD (ABP 798 vs rituximab RP)‡ (%) | 7.7 | |
| One-sided 95% lower confidence limit (%) | -1.4 | |
| One-sided 95% upper confidence limit (%) | 16.8 | |
| Two-sided 95% CI (%) | (-3.2–18.6) | |
| Best overall response, n (%) | ||
| Complete response | 29 (23.6) | 32 (25.8) |
| Unconfirmed complete response | 0 (0.0) | 3 (2.4) |
| Partial response | 67 (54.5) | 52 (41.9) |
| Stable disease | 23 (18.7) | 35 (28.2) |
| Relapsed disease | 0 (0.0) | 0 (0.0) |
| Progressive disease | 1 (0.8) | 0 (0.0) |
| Missing | 2 (1.6) | 2 (1.6) |
| Unknown | 1 (0.8) | 0 (0.0) |
The AEs observed during the study were consistent with the historical safety profile of rituximab RP [2]. A summary can be found in Table 3 [19]. Overall, similar proportions of patients experienced an AE of interest (ABP 798: 49.2% vs rituximab RP: 45.2%), grade ≥3 AEs (ABP 798: 10.9% vs rituximab RP: 10.3%) and SAEs (ABP 798: 3.9% vs rituximab RP: 4.0%) [19]. In addition, discontinuation of drug or study due to one or more AEs occurred in four patients (3.1%) in the ABP 798 group and one patient (0.8%) in the rituximab RP group. The level of ADAs was comparable within the groups.
| ABP 798 N = 128 n (%) | Rituximab RP N = 126 n (%) | |
|---|---|---|
| Any adverse event | 107 (83.6) | 95 (75.4) |
| Treatment-emergent events in ≥5% of patients | ||
| Abdominal pain | 4 (3.1) | 10 (7.9) |
| Asthenia | 12 (9.4) | 6 (4.8) |
| Diarrhea | 3 (2.3) | 9 (7.1) |
| Fatigue | 13 (10.2) | 12 (9.5) |
| Headache | 15 (11.7) | 12 (9.5) |
| Nausea | 6 (4.7) | 14 (11.1) |
| Pruritus | 6 (4.7) | 12 (9.5) |
| Pyrexia | 8 (6.3) | 8 (6.3) |
| Rash | 9 (7.0) | 6 (4.8) |
| Throat irritation | 9 (7.0) | 8 (6.3) |
| Upper respiratory tract infection | 7 (5.5) | 1 (0.8) |
| Urticaria | 7 (5.5) | 2 (1.6) |
| Grade ≥3 adverse events | 14 (10.9) | 13 (10.3) |
| Any AEOI | 63 (49.2) | 57 (45.2) |
| Infusion reactions including hypersensitivity† | 55 (43.0) | 54 (42.9) |
| Hematological reactions | 7 (5.5) | 6 (4.8) |
| Cardiac disorders | 3 (2.3) | 2 (1.6) |
| Serious infections | 2 (1.6) | 0 (0.0) |
| Severe mucocutaneous reactions | 1 (0.8) | 0 (0.0) |
| Gastrointestinal perforation, hepatitis B reactivation, opportunistic infection, progressive multifocal leukoencephalopathy, reversible posterior leukoencephalopathy syndrome, tumor lysis syndrome | 0 (0.0) | 0 (0.0) |
In addition, the PK and PD of ABP 798 and rituximab RP were evaluated in patients with follicular lymphoma [19]. Similarity of clinical PK was assessed by trough serum concentrations as well as concentrations after the end of infusion at week 12 that were similar across the ABP 798 and rituximab RP groups (Figure 5) [19]. Similarity of PD between ABP 798 and rituximab RP was also demonstrated by a comparable percentage of patients with complete depletion of CD19+ cell count at day 8.

Note: This graph represents pharmacokinetic results from the 45 patients treated with ABP 798 and the 41 patients treated with rituximab reference product who agreed to the optional pharmacokinetic sampling. The sample sizes for each visit (ABP 798 vs rituximab) were as follows: week 4 predose (44 vs 39), week 4 post-dose (36 vs 32), week 5 (38 vs 37) and week 12 predose (44 vs 41).
Reproduced with permission from [19] under the terms of the Creative Commons CC BY-NC license.
These clinical results, in two different indications that span malignant and autoimmune disease, contribute to the TOE that supports the conclusion that there are no clinically meaningful differences between ABP 798 and rituximab RP.
Extrapolation
Extrapolation is the approval of a biosimilar drug for an indication held by the RP without direct studies of the biosimilar for that indication [9]. Thus extrapolation may reduce or eliminate the need for duplicative clinical studies of a biosimilar in multiple indications and expedite regulatory approval. The scientific justification for extrapolation is based on the TOE generated during biosimilar development, including analytical and functional, non-clinical and clinical data.
Although the approval of a candidate biosimilar may be extrapolated to indications that share a MOA, this was more challenging for ABP 798 because rituximab RP is indicated for a broad range of oncological and autoimmune diseases. In addition to differences in disease pathophysiology, RA and NHL patient populations are quite distinct and expected to have different comorbidities and comedications. For example, RA patients have active inflammation and are likely to be taking an immunosuppressant such as methotrexate [43]. Moreover, the posology differs between RA and NHL: the rituximab RP dose for RA patients is two 1000 mg intravenous infusions separated by 2 weeks every 24 weeks, while NHL patients are dosed based on their body surface area [2]. These differences can lead to different PK and immunogenicity profiles. Thus, to demonstrate that there are no clinically meaningful differences between ABP 798 and rituximab RP, comparative clinical studies were conducted in both RA and NHL patient populations. The approval of ABP 798 for use in other oncology indications for which rituximab RP is approved (e.g., chronic lymphocytic leukemia and diffuse large B-cell lymphoma) is based on extrapolation. This is justifiable because ABP 798 has the same receptor target and MOA across B-cell malignancies and no clinically meaningful differences in PK/PD, immunogenicity or other safety risks. Similarly, other autoimmune indications for which rituximab RP is approved (e.g., granulomatosis with polyangiitis and microscopic polyangiitis) are based on extrapolation.
Discussion
In this review we have described the TOE that supported the regulatory approval of ABP 798 as a biosimilar to the rituximab RP in the USA and Canada. Based on comparative analytical assessment, ABP 798 was found to be highly similar to rituximab RP in structure and function, including ADCC, CDC, ADCP and apoptosis activities. The clinical efficacy of ABP 798 and rituximab RP are primarily mediated through the induction of these effector functions.
Clinical PK/PD of ABP 798 and rituximab RP were also similar. In addition, comparative clinical studies in two of the main indications of the RP – moderate-to-severe RA and low-tumor-burden follicular lymphoma – demonstrated no clinically meaningful differences in efficacy or safety. The homogeneous populations within each study, as well as the selection of sensitive end points (DAS28-CRP for RA and ORR for NHL), provided a direct measure for comparing the activity of ABP 798 with that of rituximab RP.
From scientific, resource allocation and ethical perspectives, the development of a biosimilar should not seek to replicate the clinical data of the RP across all indications. In accordance with regulatory requirements, the approval of ABP 798 in some indications of rituximab RP was based on the extrapolation of data collected in NHL and RA studies, plus a scientific justification related to the consistency of MOA across indications. Extrapolation to indications for which comparative clinical studies of ABP 798 with rituximab RP were not conducted (e.g., chronic lymphocytic leukemia, diffuse large B-cell lymphoma, granulomatosis with polyangiitis, and microscopic polyangiitis) was scientifically justified based on the strength of the TOE, the similarity of disease pathophysiology and the consistency of the MOA of rituximab RP across CD20-expressing lymphoid malignancies and autoimmune diseases.
With healthcare costs continuing to rise, biosimilars may have a robust impact on costs for healthcare systems and consumers alike [44,45]. For instance, a recent analysis found that rituximab biosimilars are associated with savings of approximately 40% compared with the RP [44]. In addition, the emergence of biosimilars may facilitate expanded worldwide access to biologics. The use of rituximab biosimilars has become increasingly well established since the approval of rituximab-abbs (TRUXIMA®; Celltrion, Incheon, South Korea) in 2018 and rituximab-pvvr (RUXIENCE®; Pfizer, NY, USA) in 2019 [46,47]. ABP 798 offers another treatment option for patients. Real-world studies can provide insights into different aspects of treatment and patient outcomes to enhance the evidence generated from clinical studies. For example, a real-world study of rituximab-abbs found no significant differences in ORR, complete response rate, progression-free survival and overall survival of patients with diffuse large B-cell lymphoma, compared with rituximab RP [48]. Similarly, an evaluation of medical chart data for patients with NHL from UK routine clinical practice demonstrated that rituximab-abbs has real-world response rates, survival and tolerability profile comparable to those of rituximab RP [49]. Moreover, a real-world study in which rituximab RP patients with RA were switched to rituximab-abbs noted similar efficacy 4 months post-switch [50]. These real-world data further increase the evidence supporting the clinical efficacy of rituximab biosimilars in autoimmune and oncology indications. Furthermore, no new safety concerns have been identified.
Conclusion
The CD20-targeted monoclonal antibody rituximab RP is a biological medicine widely used for the treatment of RA and lymphoid malignancies. Biosimilars are evaluated using state-of-the-art techniques that compare a candidate biosimilar with the RP. ABP 798 was found to be highly similar to rituximab RP based on a comprehensive TOE which comprised comparative analytical and non-clinical studies as well as two comparative clinical trials: one in moderate-to-severe RA and one in low-tumor-burden follicular lymphoma. No clinically meaningful differences in safety or effectiveness were observed between the two products. The TOE for ABP 798 also supports justification for use in indications for which rituximab RP has been approved. Rituximab biosimilars have been integrated into clinical practice with the potential to provide greater access to these costly, yet highly effective, biological medicines.
Future perspective
Over the last 15 years, biological medicines have drastically improved patient outcomes and become the standard of care for many conditions, including autoimmune and inflammatory diseases and cancer; however, their high cost has driven up healthcare expenditures and prevented some patients from having access to these important therapeutics. More recently, dozens of biosimilar medicines – defined as biologics which are highly similar to the originator or RP with no clinically meaningful differences – have been approved and successfully used in clinical practice with no evidence that they perform any differently than the originator biological therapies. With the approaching expiration of exclusivity periods for many originator biologics, more biosimilars will be approved by regulatory authorities within the next 5–10 years. As healthcare professionals’ knowledge and real-world clinical experience with biosimilars grows, it is expected that these therapies will play an increasingly important role in the future healthcare landscape. It is our opinion that the growth of biosimilars will be supported by a higher demand for healthcare as the global population grows and ages while payors simultaneously urge cost reduction. Moreover, it is also hoped that accumulating real-world evidence in the coming years will provide further assurance that biosimilars offer a safe and effective alternative to originator biologics and will lead to full integration of biosimilars for the treatment of autoimmune and inflammatory conditions and cancer. Although it is unlikely that biosimilar price reductions will be on par with those observed with generic drugs, it is anticipated that potential lower costs will benefit global healthcare systems and offer more patients access to biological medicines without compromising safety, efficacy or quality.
Biosimilar development
Biosimilars are biological medicines with no clinically meaningful differences in safety or efficacy from licensed reference products (RPs).
The regulatory approval pathway for biosimilars is based on the totality of evidence which uses a stepwise investigational approach that aims to demonstrate a high degree of similarity between a biosimilar candidate and its RP.
ABP 798, a rituximab biosimilar
ABP 798 is a biosimilar to rituximab RP, a chimeric mouse/human monoclonal IgG1κ antibody that is indicated for the treatment of a number of lymphoid malignancies and autoimmune diseases.
Totality of evidence for ABP 798
ABP 798 was found to be highly similar to rituximab RP based on a comprehensive totality of evidence which comprised comparative analytical and functional clinical studies as well as two comparative clinical trials, one in moderate-to-severe rheumatoid arthritis and one in low-tumor-burden follicular lymphoma.
No clinically meaningful differences in safety or effectiveness were observed between the two products.
‡Licensing disclosure
At the time of this publication, RIABNI™ has been approved by the US FDA for the treatment of adult patients with non-Hodgkin lymphoma, chronic lymphocytic leukemia, granulomatosis with polyangiitis, and microscopic polyangiitis. Please consult the product’s approved label for information regarding country-specific approved uses for RIABNI.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Financial & competing interests disclosure
Open access and accelerated publication fees for this review were funded by Amgen Inc., Thousand Oaks, CA. The ABP 798 studies described in this review were sponsored by Amgen Inc. S Lehto, N Seo and V Hanes are or were employees of Amgen at the time of this work and hold Amgen stock. S Cohen reports research grants from AbbVie, Amgen, Pfizer, Eli Lilly, Genentech, Roche and Bristol Myers Squibb; consulting fees from Pfizer, Eli Lilly, Genentech and Bristol Myers Squibb; honoraria from Pfizer for a speakers’ bureau; and participation in an advisory board for Gilead Sciences. C Hamm reports consulting fees from Kite, Pfizer, and Segan; and grants from Apotex. G Burmester reports consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos NV, Merck Sharp & Dohme, Pfizer and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by A Romero, Amgen, Inc., under the guidance of S Lehto, Amgen, Inc.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv. Ther. 34(10), 2232–2273 (2017). • Comprehensive overview of rituximab for B-cell hematological malignancies.
- 2. Genentech. Rituxan® (rituximab) prescribing information. www.gene.com/download/pdf/rituxan_prescribing.pdf
- 3. RIABNI™ (rituximab-arrx) Prescribing Information, Amgen Inc. (December 2020). www.pi.amgen.com/united_states/riabni/riabni_pi_english.pdf
- 4. . Rituximab: mechanism of action. Semin. Hematol. 47(2), 115–123 (2010).
- 5. . Internalization of rituximab and the efficiency of B Cell depletion in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheumatol. 67(8), 2046–2055 (2015).
- 6. RIABNI™ Product Monograph. Amgen Canada Inc (March 11, 2021). www.amgen.ca/products/
- 7. US Food and Drug Administration. Biosimilars action plan: balancing innovation and competition (July 2018). www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/UCM613761.pdf
- 8. European Medicines Agency. Guideline on similar biological medicinal products (CHMP/437/04 Rev. 1) (23 October 2014). www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf
- 9. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product (April 2015). www.fda.gov/media/82647/download •• Key regulatory guidance document from the US FDA outlining the requirements and scientific justifications for biosimilar approval.
- 10. US Food and Drug Administration. Implementation of the Biologics Price Competition and Innovation Act of 2009 (12 February 2016). www.fda.gov/drugs/guidance-compliance-regulatory-information/implementation-biologics-price-competition-and-innovation-act-2009
- 11. . Biosimilars: what the oncologist should know. Future Oncol. 15(10), 1147–1165 (2019).
- 12. . Biosimilarity versus manufacturing change: two distinct concepts. Pharm. Res. 33(2), 261–268 (2016).
- 13. . Manufacturing of biologics. In: Biologic and Systemic Agents in Dermatology. Yamauchi P (Ed.). Springer, Cham, Switzerland (2018).
- 14. World Health Organization. Guidelines on evaluation of similar biotherapeutic products (SBPs). WHO Technical Report Series No. 977 (2013). https://www.who.int/biologicals/publications/trs/areas/biological_therapeutics/TRS_977_Annex_2.pdf • Important guidance from WHO outlining globally acceptable principles for biosimilar development.
- 15. US Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product guidance for industry (April 2015). www.fda.gov/media/135612/download
- 16. . Developing the totality of evidence for biosimilars: regulatory considerations and building confidence for the healthcare community. BioDrugs 31(3), 175–187 (2017). •• Comprehensive review of the step-wise approach to developing biosimilars and the concept of totality of evidence to support regulatory approval.
- 17. European Medicines Agency, Committee for Medicinal Products for Human Use. Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues (2012). www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf •• Key regulatory guidance document from the EMA outlining the requirements for producing biosimilars.
- 18. Analytical and functional similarity of biosimilar ABP 798 with rituximab reference product. Biologicals 68, 79–91 (2020).
- 19. Efficacy and safety of ABP 798: results from the JASMINE trial in patients with follicular lymphoma in comparison with rituximab reference product. Target. Oncol. 15(5), 599–611 (2020).
- 20. Non-clinical similarity of biosimilar ABP 798 with rituximab reference product. Biologicals 72, 42–53 (2021).
- 21. . A randomized, double-blind study comparing pharmacokinetics and pharmacodynamics of proposed biosimilar ABP 798 with rituximab reference product in subjects with moderate to severe rheumatoid arthritis. Clin. Pharmacol. Drug Dev. 9(8), 1003–1014 (2020).
- 22. . Efficacy and safety results from a randomized double-blind study comparing proposed biosimilar ABP 798 with rituximab reference product in subjects with moderate-to-severe rheumatoid arthritis. Clin. Rheumatol. 39(11), 3341–3352 (2020).
- 23. . The process defines the product: what really matters in biosimilar design and production? Rheumatology (Oxford) 56(Suppl. 4), iv14–iv29 (2017).
- 24. . The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood 116(19), 3705–3714 (2010).
- 25. . Current perspective on rituximab in rheumatic diseases. Drug Des. Devel. Ther. 11, 2891–2904 (2017).
- 26. A novel method for determining antibody-dependent cellular phagocytosis. J. Immunol. Methods 468, 55–60 (2019).
- 27. . B cell depletion therapies in autoimmune disease: advances and mechanistic insights.Nat Rev Drug Discov.20(3), 179–199 (2021). • Excellent overview of B-cell biology and B-cell depletion therapies for autoimmune diseases.
- 28. Arthritis Rheum. 62(9), 2569–81 (2010).
- 29. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350(25), 2572–2581 (2004).
- 30. Continued inhibition of structural damage over 2 years in patients with rheumatoid arthritis treated with rituximab in combination with methotrexate. Ann. Rheum. Dis. 69(6), 1158–1161 (2010).
- 31. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54(9), 2793–2806 (2006).
- 32. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)). Ann. Rheum. Dis. 69(9), 1629–1635 (2010).
- 33. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 54(5), 1390–1400 (2006).
- 34. Efficacy and safety of retreatment in patients with rheumatoid arthritis with previous inadequate response to tumor necrosis factor inhibitors: results from the SUNRISE trial. J. Rheumatol. 37(5), 917–927 (2010).
- 35. Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a Phase III randomized study (MIRROR). Rheumatology (Oxford) 49(9), 1683–1693 (2010).
- 36. . The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin. Exp. Rheumatol. 34(101 Suppl. 5), S40–S44 (2016).
- 37. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364(9430), 263–269 (2004).
- 38. DAS28 best reflects the physician’s clinical judgment of response to infliximab therapy in rheumatoid arthritis patients: validation of the DAS28 score in patients under infliximab treatment. Arthritis Res. Ther. 7(5), R1063–1071 (2005).
- 39. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J. Clin. Oncol. 25(15), 1986–1992 (2007).
- 40. . Role of antibody-based therapy in indolent non-Hodgkin’s lymphoma. Leuk. Res. Rep. 16, 100275 (2021).
- 41. . Follicular lymphoma: 2018 update on diagnosis and management. Am. J. Hematol. 93(2), 296–305 (2018).
- 42. . Follicular lymphoma: updates for pathologists. J. Pathol. Transl. Med. 56(1), 1–15 (2022).
- 43. . Novel treatment strategies in rheumatoid arthritis. Lancet 389(10086), 2338–2348 (2017).
- 44. . The economic impact of biosimilars in oncology and hematology: the case of trastuzumab and rituximab. Anticancer Res. 39(7), 3971–3973 (2019).
- 45. . Financial toxicity and cancer treatments: help from biosimilars – the explanatory case of bevacizumab. Eur. J. Cancer 143, 40–42 (2021).
- 46. . Real-world use and acceptance of biosimilar monoclonal antibodies of rituximab in oncology practice in the USA. Future Oncol. 17(30), 3941–3950 (2021).
- 47. . Real-world use and acceptance of rituximab biosimilars in non-Hodgkin lymphoma in an oncologist network in Germany. Future Oncol. 16(15), 1001–1012 (2020).
- 48. The clinical outcomes of rituximab biosimilar CT-P10 (Truxima) with CHOP as first-line treatment for patients with diffuse large B-cell lymphoma: real-world experience. Leuk Lymphoma. 61(7), 1575–1583 (2020).
- 49. Assessment of rituximab-abbs, a biosimilar, and rituximab outcomes in patients with CLL or NHL: a real-world UK study. Leuk. Res. 111, 106671 (2021).
- 50. Real-world experience of effectiveness of non-medical switch from originator to biosimilar rituximab in rheumatoid arthritis. Rheumatology (Oxford) 60(8), 3679–3688 (2021).

































