Adverse events in women switching from olaparib capsules to tablets: retrospective observational study of US claims data
Abstract
Aim: Describe rates of prespecified adverse events in patients who switched from olaparib capsules to tablets. Patients & methods: Retrospective, observational cohort analysis using self-controlled, pre–post design. Data on patients with ovarian cancer who switched from olaparib capsules to tablets between January 2015 and February 2019 were obtained from a US claims database. Results: Among all patients (n = 48), proportion with any prespecified adverse event was 45.8% (95% confidence interval: 31.4–60.8) during initial 90 days’ capsule use and 35.4% (22.2–50.5) during initial 90 days’ tablets use; difference -10.4% (-28.8–9.0). Conclusion: Switching from olaparib capsules to tablets was manageable with no evidence of increased toxicity. This real-world study supports the manageable tolerability of olaparib in women with ovarian cancer.
Olaparib, the first approved PARP inhibitor, led to a paradigm shift in the standard of care for women with BRCA-mutated (BRCAm) ovarian cancer. Olaparib (Lynparza®) is now an established therapy, as first-line maintenance and treatment for recurrent disease in women with BRCAm advanced ovarian cancer, and as maintenance therapy in platinum-sensitive, recurrent advanced ovarian cancer irrespective of BRCA mutation status.
Olaparib was first approved by the US FDA in December 2014 as a capsule (400 mg twice daily [bid]) for the treatment of women with germline BRCAm recurrent advanced ovarian cancer after ≥3 lines of therapy and by the EMA in October 2014 for the maintenance treatment of BRCAm platinum-sensitive relapsed ovarian cancer [1,2]. The high pill burden of the capsule formulation (16 capsules/day) has practical drawbacks for patients and there is an unmet need for a more convenient maintenance therapy in ovarian cancer to encourage patient compliance [3]. A tablet formulation of olaparib, offering a reduced pill burden and a more convenient dosing regimen (300 mg bid; four tablets/day), has been developed. This was licensed by the FDA in August 2017 [4,5] and by the EMA in May 2018 [6,7].
The first clinical evidence of olaparib efficacy in ovarian cancer patients used the capsule formulation (Study 42; Study 19) [8–10]. Following development of the olaparib tablet formulation, an adaptive pharmacokinetic/dose-finding study determined that the optimal olaparib tablet dose for evaluation in Phase III trials was 300 mg bid (two 150 mg tablets twice a day) [11]. A Phase III study, SOLO2, confirmed the efficacy and tolerability of olaparib tablets as a maintenance treatment in patients with platinum-sensitive BRCAm recurrent ovarian cancer [12]. Despite dosing and bioavailability differences between olaparib capsules and tablets, consistency of clinical outcomes between the 400 mg bid capsule dose and the 300 mg bid tablet dose suggest similar benefit–risk profiles [13,14]. Adverse events (AEs) appear comparable; for example, nausea and vomiting are generally low grade and manageable for both formulations [9,12–15].
The capsule and tablet doses are not interchangeable, due to differences in bioavailability [11], and physicians are advised to adhere strictly to the dosing instructions provided in the prescribing information when transitioning patients from capsules to tablets [5,7,14]. Practical guidelines recommend that patients switching from olaparib capsules to tablets should be monitored closely for the first 3 months and their dose adjusted according to tolerability; particular care should be taken when transitioning patients who are prescribed capsules below the licensed starting dose [14].
Real-world data evaluating the tolerability profile of the olaparib tablet formulation among patients who have switched from capsules are limited [16]. Capsules were voluntarily withdrawn from the US market in September 2018 (3 years and 9 months after FDA approval), at which point any US patient on olaparib capsules switched to tablets. Here, we utilize a large US health insurance claims database to characterize the tolerability profile of a cohort of ovarian cancer patients who switched from olaparib capsules to tablets. This study aims to describe the pattern of prespecified AEs experienced in this group.
Patients & methods
Design
This was a retrospective, observational cohort analysis using a self-controlled, pre–post design. A pre–post study measures the occurrence of an outcome before and again after a particular intervention is implemented. A self-controlled design was selected as we are specifically interested in the subset of patients who switch olaparib formulations from capsules to tablets.
The primary objective was to describe the incidence of prespecified AEs among qualifying patients who transitioned from olaparib capsules to tablets. These AEs were chosen due to their reported frequency in olaparib clinical trials [9,12] or their clinical importance (see below for further details). Secondary objectives were to describe: patient demographic characteristics at the index date; clinical characteristics and key comorbidities at the index date; treatment patterns and line of therapy metrics, including dosing and duration metrics, platinum status and antiemetic use.
Further analyses were performed to characterize the tolerability profile of olaparib in long-term capsule responders and evaluate whether dose reduction while on capsules was associated with similar dose adjustments upon initiation of tablets. Long-term capsule responders were defined as ovarian cancer patients prescribed olaparib capsules for ≥12 months and prespecified AEs were stratified by time on capsule (capsule exposure 12–24 months vs >24 months). Dosing outcomes while on tablets were assessed among patients who had been prescribed capsules below the licensed dose at the time of switching.
Data acquisition & study population
Symphony Health (https://symphonyhealth.prahs.com/) was contracted to support this research. Treatment, medical and hospital claims data were obtained from the Symphony Health’s US Patient Integrated Dataverse database. The database includes open, unadjudicated claims submitted to all payer types (commercial plans, Medicare Part D, cash, assistance programs, Medicaid). The majority of pharmacy claims across retail, specialty and mail-order channels, and 60% of professional medical outpatient claims, are captured. Pharmacy claims are adjudicated; paid claims and medical claims are unadjudicated. Data are deidentified, in compliance with the Health Insurance Portability and Accountability Act (HIPPA).
Eligible patients had confirmed epithelial ovarian, fallopian tube or primary peritoneal cancer based on International Classification of Diseases (ICD) 9/10 code criteria, and had switched from a prescription of olaparib capsules to tablets between January 2015 and February 2019. The identification period was January 2012 to February 2019. The index date was defined as the time of the first olaparib capsule prescription. Patients were required to have at least 90 days of follow-up after the index date, and at least 90 days of follow-up after switching to tablets. Patients must have had relevant healthcare activity (i.e., visited physicians and pharmacies) captured by consistently reporting physicians for at least 80% of the study period, herein defined as meeting eligibility and stability criteria, respectively (Figure 1). Patients were excluded if they had a prior history of primary tumor other than ovarian, fallopian tube or primary peritoneal cancer (patients with BRCAm breast cancer were excluded), or if they received olaparib capsules after tablets. Olaparib treatment that immediately followed chemotherapy was defined as maintenance therapy.

†Patient’s relevant healthcare activity observed.
‡Patient’s healthcare activity is captured from consistent reporting physicians.
Outcome variables: AEs & dosimetry
Eight prespecified AEs were selected for evaluation: anemia, fatigue, nausea, vomiting and diarrhea (based on frequency in previous olaparib clinical trials) [9,12], together with neutropenia, leukopenia and thrombocytopenia (based on clinical importance). Fatigue, nausea, vomiting, anemia and diarrhea are the most frequently reported AEs of any grade in patients receiving olaparib; instances are generally of mild or moderate intensity (grade 1 or 2) [1,5,7].
Prespecified AEs were identified from healthcare claims data based on ICD 9/10 code criteria (
Exposure variables
Treatment duration of capsules was defined as the total number of days’ supply patient had without a gap of 30 days or more before switching to tablets. Treatment duration of tablets was defined as the total number of days’ supply patient had without a gap of 30 days or more. Dose was calculated as (quantity dispensed x strength)/days’ supply. Starting dose, treatment duration, dose at 90 days and dose reductions on capsules, and separately on tablets, were assessed.
Statistical analyses
Participant characteristics at baseline are reported using mean (standard deviation), percentage (number) and/or median (range), depending on the distribution of underlying variables. The proportion of patients with prespecified AEs (percentage and associated 95% Clopper-Pearson [“exact”] confidence interval [CI]) [17] are reported separately for the first 90 days following olaparib capsule and tablet therapy. The difference in proportions is presented as ‘tablets minus capsules’ (with 95% CI using the Wilson score method): [18] negative values indicate that the incidence of prespecified AEs is lower with tablets than with capsules. These analyses are produced for all patients with the prespecified AE and within the two subgroups defined by whether or not the patient experienced the prespecified AE in the 12 months prior to initiating olaparib (the baseline period).
The study included all patients in the database who met the inclusion and exclusion criteria. A sample size of 50 patients allows the incidence of a prespecified AE to be estimated with a precision of approximately ± 15%. Repeated measures logistic regression evaluated whether experiencing a prespecified AE varied over three time periods; baseline (12 months before olaparib), post capsules or tablet.
Sensitivity analyses were carried out to test the robustness of estimates. The first analysis included a larger population of ovarian cancer patients (n = 103) who had switched olaparib formulation and had 90 days of follow-up on capsules and tablets, but for whom baseline data were not available. The second analysis reduced the pre-olaparib initiation baseline period to 90 days and reported multilevel logistic regression of ‘any prespecified AE’.
Data were analyzed using R. [19].
Results
Study population
Of 7468 identified patients in the Dataverse database with at least one prescription for olaparib between January 2015 and February 2019, 123 were qualifying ovarian cancer patients who had switched formulation from olaparib capsules to tablets (Figure 1). Of these, 48 patients met stability criteria (at least a 90-day supply of both olaparib capsules and tablets, and healthcare activity captured from consistent reporting physicians) and form the main study cohort (Figure 1).
Among the 48 ovarian cancer patients included in the primary analysis, mean (standard deviation) age at initiation of olaparib was 61 (8) years, 67% (n = 32) were commercially insured and 79% (n = 38) had received olaparib capsule treatment for >1 year before switching to tablets (Table 1). A quarter of patients (n = 12) had received platinum-based chemotherapy in the 18 weeks prior to initiating olaparib capsule therapy. Just over a third (35% [n = 17]) of those who switched formulations had no day’s gap between stopping capsules and starting tablets (
| Characteristic | Primary analysis group (n = 48) |
|---|---|
| Age, median (range) years at index date | 61 (55–68) |
| Age category at index date, n (%), years – ≤49 – 50–59 – 60–69 – 70–79 – ≥80 | 5 (10) 15 (31) 20 (42) 8 (17) 0 |
| Payer type, n (%) – Commercial – Medicare – Medicaid – Other | 32 (67) 13 (27) 2 (4) 1 (2) |
| Index year, n (%) – 2015 – 2016 – 2017 – 2018 | 14 (29) 15 (31) 18 (38) 1 (2) |
| Time from initial diagnosis to index date, median (range) years | 2.78 (2.07–4.28) |
| Number of metastatic sites prior to or on the index date, n (%) – 1 – 2 – 3 – 4+ | 16 (33) 4 (8) 6 (13) 5 (10) |
| BRCA testing status, n (%) – Yes – No, unknown, or missing | 11 (23) 37 (77) |
| Prior chemotherapy treatments (proxy for maintenance), n (%) – Platinum in 18 weeks prior to olaparib capsule – Bevacizumab in 2 years prior to olaparib capsule | 12 (25) 9 (19) |
| Length of time between capsules and tablets, n (%) – <6 months – 6 months to 1 year – >1 year | 1 (2) 9 (19) 38 (79) |
AE frequency in the first 90 days of olaparib capsule/tablet therapy
The incidence of the eight prespecified AEs was broadly similar for capsules and tablets during the 90 days following olaparib initiation, regardless of formulation (Table 2). The most commonly reported prespecified AEs during the first 90 days of capsule and tablet use were anemia (19 and 25% of patients, respectively) and fatigue (8% in both groups). Neutropenia and thrombocytopenia were, respectively, reported by 2% (n = 1) and 4% (n = 2) of patients during the first 90 days of capsule use, compared with 6% (n = 3) each during the first 90 days of tablet use. The incidences of vomiting, nausea and diarrhea were all low (<5%) during the first 90 days of capsule and tablet use. Antiemetic treatment was reported for 25% (n = 12) patients during capsule use and 13% (n = 6) patients during tablet use. Only one instance of leukopenia was reported during the first 90 days of capsule use; this prespecified AE was therefore included in the switch analysis but is not individually itemized in all places throughout the results.
| Outcome | Analysis period (n = 48) | Time to AE post switch, median days (n = 48) | ||
|---|---|---|---|---|
| 1-year precapsule BASELINE | Within 90 days of starting olaparib CAPSULES | Within 90 days of starting olaparib TABLETS | ||
| Any prespecified AE†, n (%) | 28 (58) | 22 (46) | 17 (35) | 27 |
| – Anemia | 17 (35) | 9 (19) | 12 (25) | 13 |
| – Fatigue | 11 (23) | 4 (8) | 4 (8) | 64 |
| – Neutropenia | 5 (10) | 1 (2) | 3 (6) | 13 |
| – Thrombocytopenia | 4 (8) | 2 (4) | 3 (6) | 65 |
| – Vomiting | 9 (19) | 1 (2) | 2 (4) | 39 |
| – Nausea | 11 (23) | 1 (2) | 1 (2) | 31 |
| – Diarrhea | 4 (8) | 1 (2) | 0 | NA |
| – Leukopenia | 0 | 1 (2) | 0 | NA |
| Antiemetic use, n (%) | NA | 12 (25) | 6 (13) | 30 |
| Any hematological AE, n (%) | 18 (38) | 11 (23) | 13 (27) | 13 |
| Any nonhematological AE†, n (%) | 21 (44) | 15 (31) | 11 (23) | 30 |
Median (interquartile range [IQR]) time to any prespecified AE after switching formulation was 27 (9–39) days. Hematological AEs had a median (IQR) time to onset of 13 (9–31) days, while nonhematological AEs had a median (IQR) time to onset of 30 (18–51.5) days.
The proportion of patients with any prespecified AE during the first 90 days of capsule and tablet use was 45.8% (95% CI: 31.4–60.8) and 35.4% (95% CI: 22.2–50.5) respectively; the difference was -10.4% (95% CI: -28.8–9.0). Altogether, 28 patients had a prespecified AE during the baseline period before starting olaparib. For this group, the difference in the proportion of patients with any prespecified AE during the first 90 days of capsule and tablet use was -21.4% (95% CI: -43.6–4.4).
The proportion of patients with any prespecified hematological AE during the first 90 days of treatment was 22.9% (95% CI: 12.0–37.3) for capsules, and 27.1% (95% CI: 15.3–41.8) for tablets (difference 4.2% [-13.0–21.1]). The proportion of patients with any prespecified nonhematological AE during the first 90 days of treatment was 31.3% (95% CI: 18.7–46.3) for capsules, and 22.9% (95% CI: 12.0–37.3) for tablets (difference -8.3% [-25.4, 9.4]).
The likelihood of experiencing any prespecified AE during the initial 90 days of therapy was similar with capsules and tablets, and not statistically associated with experiencing the given AE prior to starting olaparib (Figure 2). Repeated measures logistic regression confirmed no significant difference in proportion of patients with any prespecified AE by exposure period (95% CI log odds ratio for having an AE during time on capsules versus tablets; baseline versus capsules; baseline versus tablets all overlapping 1; data not shown).
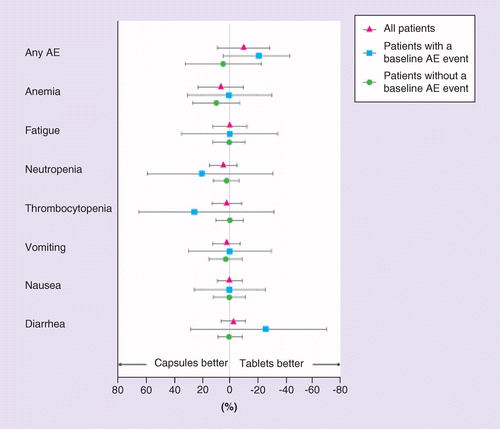
Results are plotted for all patients (red) and stratified by whether the patient experienced the relevant AE in the baseline (pre-olaparib; blue) period or not (green). Negative values indicate that the incidence was lower for tablets compared with capsules. As only one instance of leukopenia was reported, this prespecified AE is not itemized here.
AE: Adverse event.
Dosimetry
Median (range) olaparib duration of exposure in this cohort was 447 (321–830) days for capsules and 218 (163–276) days for tablets. Most patients initiated olaparib capsules and tablets at the licensed starting dose (Table 3; capsules 88% [n = 42]; tablets 81% [n = 39]). 31 patients had a gap of at least 1 day between stopping capsules and starting tablets (median treatment gap 11 days).
| Dosing metric | Primary analysis (n = 48) | Sensitivity analysis (n = 103) | ||
|---|---|---|---|---|
| Capsules | Tablets | Capsules | Tablets | |
| Starting dose, median (range) mg | 800 (800–800) | 600 (400–600) | NA | NA |
| Ending dose, median (range) mg | 800 (500–800) | 600 (400–600) | NA | NA |
| Number of dose changes during treatment, n (%) – 0 – 1 – 2 – 3 | 27 (56) 13 (27) 7 (15) 1 (2) | 42 (88) 5 (10) 1 (2) 0 (0) | NA | NA |
| Proportion with licensed starting dose, % (SD) | 87.5 (4.8) | 81.3 (5.6) | 86.4 (3.4) | 74.8 (4.3) |
| Proportion with dose changes within 90 days, % (SD) | 8.3 (4.0) | 10.4 (4.0) | 8.7 (4.0) | 14.6 (3.5) |
| Number of dose changes within 90 days, mean (SD) | 0.10 (0.37) | 0.10 (0.31) | 0.10 (0.33) | 0.15 (0.36) |
After initiation of each formulation of olaparib, a small proportion of patients had subsequent dose modifications within the first 90 days: capsules 8% (n = 4); tablets 10% (n = 5; Table 3). Almost all modifications during this period were single dose changes: three patients had one dose change while on capsules and five patients had one dose change while on tablets.
Most people received capsules for over a year (median 447 days); the proportion of patients who experienced a dose reduction during total time on capsules was 44% (n = 21). Among these 21 patients, 71% (n = 15) subsequently initiated tablets on a lower than licensed starting dose and 24% (n = 5) experienced dose changes during total time on tablets (four patients had one dose change and one patient had two dose changes). The median (range) tablet treatment duration in these 21 patients was 211 (163–268) days.
AE frequency in special populations during tablet/capsule therapy
There were 31 long-term capsule responders: 45% (n = 14) had capsule exposure of 12–24 months and 55% (n = 17) had capsule exposure >2 years. The proportion of long-term responders experiencing any prespecified AE was similar in each of these exposure groups (Table 4).
| Cohort | Number of patients with any prespecified AE | Probability of event (95% CI) | ||||
|---|---|---|---|---|---|---|
| Analysis period | On CAPSULES | On TABLETS | Difference | |||
| 1-year precapsule BASELINE | Within 90 days of starting olaparib CAPSULES | Within 90 days of starting olaparib TABLETS | ||||
| Capsule exposure 12–24 mths (n = 14) | 5 | 6 | 4 | 42.9% (17.7–71.1) | 28.6% (8.4–58.1) | -14.3% (-44.1–19.5) |
| Capsule exposure >2 years (n = 17) | 10 | 7 | 6 | 41.2% (18.4–67.1) | 35.3% (14.2–61.7) | -5.9% (-34.9–24.6) |
AE frequency in the last 90 days of capsule therapy & first 90 days of tablet therapy
There were 17 patients (35%) who switched olaparib formulations with no day’s gap between stopping capsules and starting tablets. In this group, the proportion of patients with any prespecified AE was 47.1% (95% CI: 23.0–72.2) during the last 90 days of capsule use and 41.2% (95% CI: 18.4–67.1) during the first 90 days of tablet use; the difference was -5.9% (95% CI: -25.1–35.3). The incidence of prespecified AEs was similar for capsules and tablets in the 90 days immediately before and immediately after switching (
Sensitivity analyses
Including patients who met eligibility criteria (at least a 90-day supply of both olaparib capsules and tablets, and relevant healthcare activity observed) increased the number of ovarian cancer patients eligible for analysis to 103 (Figure 1). In this larger population, the proportion of patients experiencing any prespecified AE was again similar during use of capsules 35.9% (95% CI: 26.7–46.0) and tablets 32.0% (95% CI: 23.2–42.0; Supplementary Table 3; difference -3.9% [95% CI: -16.5–8.9]). Similar to the base case, most patients in this group initiated olaparib at the licensed starting dose (capsules 86% [n = 89]; tablets 75% [n = 77], with limited dose modification (capsules 9% [n = 9]; tablets 15% [n = 15]).
Reducing the pre-olaparib baseline period from 12 months to 90 days did not qualitatively change the results. The likelihood of experiencing any prespecified AE was similar with capsules and tablets, and not influenced by having the AE prior to starting olaparib (multilevel logistic regression of ‘any AE’ 95% CI log odds ratio for having an AE during time on capsules vs tablets; 90-day baseline vs capsules; 90-day baseline vs tablets; all overlapping 1).
Discussion
Here, we utilize real-world data available from US patients who have switched from olaparib capsules to tablets, to characterize eight prespecified AEs and strengthen the existing evidence of olaparib tolerability in the ovarian cancer setting. We found that switching patients with ovarian cancer from olaparib capsules to tablets was manageable, with no evidence of increased toxicity. Many patients (35%) transitioned directly from capsules to tablets without any day’s treatment gap and without any apparent tolerability issues; importantly, these patients had a similar incidence of prespecified AEs during the last 90 days of capsule use and the first 90 days of tablet use. The reasons why most patients had a treatment break between stopping capsules and starting tablets are unclear, but the findings from our study support switching to tablets without any gap in olaparib therapy. Most patients initiated olaparib at the licensed starting dose and did not require dose modifications within the first 90 days of follow-up. Among the minority of patients with tablet dose changes during the whole follow-up period, all patients but one required a single dose adjustment.
Patients on orally administered cancer treatments can struggle to take their medication as prescribed [20], but greater treatment satisfaction is associated with better adherence [21]. Switching patients from olaparib capsules to tablets may help to improve compliance by reducing pill burden and offering a more convenient dosage regimen [3]. Switching from olaparib capsules to tablets may appear challenging for clinicians and patients, because capsule and tablet doses are not interchangeable, patients require close monitoring for AEs during the first 3 months, and dose adjustment may be required [14]. Real-world data evaluating tolerability and dosing outcomes among patients who switch from olaparib capsules to tablets are therefore desirable. Our analysis, from a real-world population of patients with ovarian cancer, offers insights to physicians transitioning their patients from olaparib capsules to tablets.
Similar findings have been reported in a real-world study in Germany [16]. Among patients with ovarian cancer treated with olaparib, 47 switched from capsules to tablets [16]. The rate of AEs was 87% before switching and 55% after switching. Treatment modifications due to AEs were more frequent during capsule treatment: 83% had therapy modifications before switching, compared with 37% after switching. The authors concluded that, under routine clinical conditions, switching from olaparib capsules to tablets was well tolerated. These findings support guidelines on managing patients who switch from capsules to tablets [14].
Prespecified AEs were selected because of their reported frequency in Phase III trials and their clinical importance. Nausea (71–77%), fatigue (63–66%), anemia (21–44%), vomiting (35–40%) and diarrhea (27–34%) are the most frequently reported AEs in patients receiving olaparib in clinical trials [12,13]. The incidence of these AEs generally appeared lower in our analysis than in clinical trials, which may reflect the limitations of retrospective claims data. For example, mild tolerability concerns are less likely to be reported by patients in clinical practice compared with a clinical trial setting. In addition, our analysis did not count multiple occurrences of an AE in the same patient. Nausea and vomiting are manageable with standard oral antiemetics [15], and our analysis showed that antiemetic use was reported for 25% of patients during capsule therapy and 13% of patients during tablet therapy.
In this study, hematological AEs, which were experienced by about a quarter of patients, occurred on average within 2 weeks of treatment initiation. Gastrointestinal AEs typically occurred after a month on therapy. Degree of prior exposure to olaparib, or previous occurrence of a specific AE, did not appear to influence the likelihood of a patient experiencing the same AE while on olaparib tablets. Patients who were long-term responders with the capsule formulation were able to transition to the tablet formulation without any additional tolerability concerns.
Current guidance is that particular care should be taken when transitioning patients who are on a reduced dose [14]. Our analyses showed that, of the 21 patients who were prescribed a reduced dose of olaparib capsules just before switching, the majority (71%) subsequently initiated tablets below the licensed starting dose [5], with little further dose adjustment.
The limitations of this study include the sample size, use of database claims data and biases inherent to observational and retrospective studies. The small sample size reduces the power to detect a difference if one exists. Claims data are captured for billing rather than research purposes, and only AEs that require medical care may be recorded: mild cases of AEs such as nausea and fatigue may be under-reported. As this is not a closed claim system, lack of access may also result in missing events. However, this is likely to be nondifferential with respect to olaparib formulation. Additional notable limitations of the claims data used here include: medical claims were unadjudicated; stage and BRCA status were not captured; it is unknown whether patients took olaparib as prescribed; dosing changes were estimated. Nevertheless, claims database studies have been used to investigate real-world AE patterns [22] and have found that the most commonly reported AEs are consistent with product labels [23]. Claims data have similar accuracy to electronic medical records [24], with the added benefit that all formulation types and dates are recorded. In our study, the overall incidence of AEs may have been underestimated for two further reasons. First, recurrent AEs, such as anemia, are a practical issue in clinical practice and only the first instance of each prespecified AE was counted in this analysis. Second, the 90-day follow-up period is relatively short. However, clinical trial data indicate that the occurrence of AEs reduces after 3 months of olaparib treatment [12], and practical recommendations support close follow-up of patients during the initial 3 months after switching [14]. Any underreporting of AEs is likely to be similar for capsules and tablets, so this is unlikely to influence the study conclusions. As with all observational analyses, residual confounding factors may bias the study estimates, despite controlling for several patient characteristics. For example, there may be some bias in favor of olaparib tablets due to the comparison between initial treatment period (with capsules) and subsequent treatment period with a different formulation (tablets). However, base-case results were supported by the sensitivity analyses, including the comparison of AEs immediately before and after switching.
The main strength of the study is the use of US patient data, as the proactive, voluntary withdrawal of olaparib capsules from the US market meant that all patients were required to switch to tablets, thereby avoiding selection bias. In addition, by comparing prespecified AEs during the first 90 days of both capsules and tablets use, we focus on the period when AEs are most likely to occur. Our findings are applicable to a US population and relevant to women with ovarian cancer in other countries who are transitioning from the olaparib capsule to tablet formulation.
This study supports that the transition from olaparib capsules to tablets is manageable, with no evidence of increased toxicity. Further research is needed to confirm these findings. The tablet formulation offers a reduced pill burden and greater convenience compared with the capsule formulation. As lower pill burden may be associated with improved quality of life in certain patient groups [25], and greater satisfaction with treatment is associated with improved adherence to oral chemotherapy [21], switching from olaparib capsules to tablets may support increased compliance. This is of particular importance for maintenance therapy [3]; poor compliance with oral chemotherapy has been shown to be associated with greater symptom severity [20].
This real-world study supports the manageable tolerability of olaparib tablets in women with ovarian cancer. From a practical point of view, it is important to reaffirm to patients that switching to olaparib tablets is similar to starting capsules and there appears to be no worsening of safety during the first 90 days after switching. Prior to implementing any changes in therapy, healthcare providers should support patients to ensure an informed transition between the formulations [14].
The capsule formulation of olaparib (Lynparza®) requires patients to take 16 capsules/day. The tablet formulation offers similar therapeutic impact with a reduced pill burden and a more convenient dosing regimen: two 150 mg tablets twice a day.
Owing to bioavailability differences, olaparib capsule and tablet doses are not interchangeable. When transitioning patients from capsules to tablets, physicians are advised to adhere strictly to the dosing instructions provided in the prescribing information, and to monitor patients closely for the first 3 months so that the dose can be adjusted if needed.
Real-world data on switching from olaparib capsules to tablets are limited, although there is some evidence that the transition is well tolerated in the clinical setting.
Using claims data obtained from a large US claims database, this retrospective, observational cohort analysis aimed to describe the incidence of prespecified adverse events among women with ovarian cancer who transitioned from olaparib capsules to tablets.
A total of 48 ovarian cancer patients who switched from a prescription for olaparib capsules to tablets between January 2015 and February 2019 were included in our primary study cohort.
Most patients (>80%) initiated olaparib (capsules or tablets) at the licensed starting dose; ≤10% of patients required dose adjustments while taking olaparib.
During the first 90 days of olaparib therapy, the proportion of patients with any prespecified AE was 45.8% (95% CI: 31.4, 60.8) while on capsules and 35.4% (95% CI: 22.2–50.5) while on tablets; the difference was -10.4% (95% CI: -28.8–9.0).
Among patients who switched olaparib formulations with no day’s gap between stopping capsules and starting tablets, the proportion with any prespecified AE was 47.1% (95% CI: 23.0–72.2) during the last 90 days of capsule use and 41.2% (95% CI: 18.4–67.1) during the first 90 days of tablet use; the difference was -5.9% (95% CI: -25.1–35.3%).
The likelihood of experiencing any prespecified adverse event during the initial 90 days of olaparib therapy was similar with capsules and tablets, and not statistically associated with experiencing the given AE prior to starting olaparib.
In conclusion, switching patients from olaparib capsules to tablets was manageable with no evidence of increased toxicity. This real-world study supports the manageable tolerability of olaparib in women with ovarian cancer.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2020-0142
Author contributions
W Sawyer and Symphony Health performed data analyses. All authors were involved in interpretation of the data, and reviewed and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript. The authors take full responsibility for the scope, direction, content of, and editorial decisions relating to the manuscript, and were involved at all stages of development. SB: ORCID ID 0000-0002-8840-7934; GHL: ORCID ID 0000-0001-5080-9885.
Acknowledgments
Data analysis was supported by Symphony Health. Dr Banerjee acknowledges the support of The National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London.
Financial & competing interests disclosure
The study and analyses were funded by AstraZeneca. S Banerjee has received honoraria or other reimbursement from Amgen, AstraZeneca, Clovis, Genmabs, Tesaro/GSK, Nucana, Roche, Merck Serono and Seattle Genetics. W Sawyer, K McLaurin, R Davidson, and GH Long are employees of AstraZeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support, in the form of the preparation and revision of the draft manuscript under the authors’ conceptual direction and based on feedback from the authors, was provided by Rebecca Sutch, PhD, on behalf of AMICULUM Ltd, UK, and was supported financially by AstraZeneca.
Ethical conduct of research
This research involves the secondary analysis of de-identified data and, as such, is exempt from both institutional review board approval and from human subject research informed consent (Federal Policy for the Protection of Human Subjects, 45 CFR 46). This report conforms with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. AstraZeneca. LYNPARZA – olaparib capsule prescribing information (2018). www.azpicentral.com/lynparza/lynparza.pdf
- 2. AstraZeneca. LYNPARZA capsules – summary of product characteristics (2019). www.medicines.org.uk/emc/product/6821/smpc
- 3. . Olaparib tablet: a review in ovarian cancer maintenance therapy. Target Oncol. 13(6), 801–808 (2018). • This review summarizes the efficacy and tolerability profile of olaparib tablets based on clinical trial data.
- 4. AstraZeneca press release. Lynparza receives additional and broad approval in the US for ovarian cancer (2017). www.astrazeneca.com/media-centre/pressreleases/2017/lynparza-receives-additional-and-broad-approval-in-the-us-for-ovariancancer-17082017.html#
- 5. AstraZeneca. LYNPARZA – olaparib tablet prescribing information (2019). www.azpicentral.com/lynparza_tb/lynparza_tb.pdf • Contains the FDA-approved indication for olaparib tablets, together with guidance regarding starting dose, dose adjustments, and tolerability.
- 6. AstraZeneca press release. Lynparza tablets receive EU approval for the treatment of platinum-sensitive relapsed ovarian cancer (2018). www.astrazeneca.com/media-centre/press-releases/2018/lynparza-tablets-receive-eu-approval-for-the-treatment-of-platinum-sensitive-relapsed-ovarian-cancer08052018.html
- 7. AstraZeneca. LYNPARZA tablets – summary of product characteristics (2019). www.medicines.org.uk/emc/product/9204/smpc • Contains the EMA-approved indication for olaparib tablets, together with guidance regarding starting dose, dose adjustments and tolerability.
- 8. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 33(3), 244–250 (2015).
- 9. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 366(15), 1382–1392 (2012). • This randomized, Phase II clinical trial examines the efficacy and tolerability of olaparib capsules as maintenance treatment in patients with platinum-sensitive, relapsed, high-grade ovarian cancer: progression-free survival was significantly longer compared with placebo.
- 10. . Olaparib maintenance for first-line treatment of ovarian cancer: will SOLO1 reset the standard of care? Future Oncol. 15(16), 1845–1853 (2019).
- 11. An adaptive study to determine the optimal dose of the tablet formulation of the PARP inhibitor olaparib. Target Oncol. 11(3), 401–415 (2016).
- 12. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet Oncol. 18(9), 1274–1284 (2017). •• This randomized, Phase III clinical trial examines the efficacy and tolerability of olaparib tablets as maintenance treatment in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation: progression-free survival was significantly longer compared with placebo.
- 13. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 379(26), 2495–2505 (2018). •• The SOLO1 randomized, Phase III clinical trial examines the efficacy and tolerability of olaparib tablets as maintenance treatment in patients with newly diagnosed, high-grade ovarian cancer and a BRCA1/2 mutation: progression-free survival was significantly longer compared with placebo.
- 14. . Practical considerations for clinicians for transitioning patients on maintenance therapy with olaparib capsules to the tablet formulation of olaparib. Asia Pac. J. Clin. Oncol. 14(6), 459–464 (2018). •• This article presents detailed considerations and recommended steps when transitioning patients from olaparib capsules to tablets.
- 15. Management of nausea and vomiting during treatment with the capsule (CAP) and tablet (TAB) formulations of the PARP inhibitor olaparib. Eur. J. Cancer 51, S550 (2015).
- 16. Results of the 3rd interim analysis of C-Patrol: a non-interventional study on olaparib in German routine clinical practice. Ann. Oncol. 30(Suppl. 5), Abstr 1007P (2019). •• This congress abstract presents real-world tolerability outcomes and dose modifications among patients transitioning from olaparib capsules to tablets in Germany.
- 17. . The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26, 404–413 (1934).
- 18. . Probable interference, the law of succession, and statistical inferance. J. Am. Stat. Assoc. 22, 209–212 (1927).
- 19. Rcoreteam. A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Version 3.6.2 (2019). www.r-project.org/
- 20. Patient experiences with oral chemotherapy: adherence, symptoms, and quality of life. J. Natl Compr. Canc. Netw. 17(3), 221–228 (2019).
- 21. Treatment satisfaction and adherence to oral chemotherapy in patients with cancer. J. Oncol. Pract. 13(5), e474–e485 (2017).
- 22. Adverse events in giant cell arteritis and rheumatoid arthritis patient populations: analyses of tocilizumab clinical trials and claims data. Rheumatol. Ther. 6(1), 77–88 (2019).
- 23. Real-world treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer 19(1), 548 (2019).
- 24. . Prediction accuracy with electronic medical records versus administrative claims. Med. Care 57(7), 551–559 (2019).
- 25. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res. Ther. 15(1), 17 (2018).
































