Neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer
Abstract
ABSTRACT
Chemoradiotherapy or short-course radiotherapy followed by surgery is a standard treatment for locally advanced rectal cancer. This multimodality strategy has reduced the risk of local recurrence but failed to improve survival. Moreover, mid- and long-term side effects of radiotherapy have been reported. Alternative strategies have been investigated in an attempt to minimize treatment-related toxicities and improve outcome. Neoadjuvant chemotherapy without radiotherapy is an attractive therapeutic option that yields theoretical advantages. Moreover, if carefully selected, patients may be spared the effects of radiotherapy without compromising the oncology outcome. The authors review the available evidence on neoadjuvant chemotherapy without radiotherapy in locally advanced rectal cancer and try to anticipate potential algorithms of treatment selection to implement in clinical practice in the future.
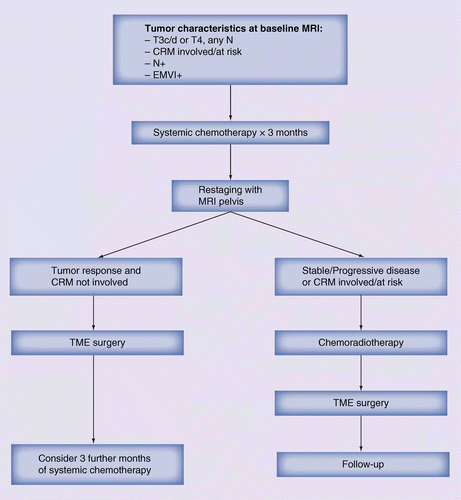
CRM: Circumferential resection margin; EMVI: Extramural venous invasion; TME: Total mesorectal excision.
Rectal cancer represents approximately a third of colorectal cancers and remains an important contributor to the global tumor burden. In the UK in 2010, 8753 new cases of rectal cancer were diagnosed in males and 5217 in females and overall rectal cancer accounted for approximately 4% of all cancer deaths, with 3608 males and 2294 females dying of this disease [1].
Over the past decades, rectal cancer mortality rates have decreased [1]. In patients with locally advanced disease (T3–4 and/or N+), the adoption of a multimodality therapy alongside important advances in diagnostics (i.e., routine use of MRI), surgery (i.e., standardization of total mesorectal excision [TME]) and pathology (i.e., routine assessment of the plane of surgery and circumferential resection margin [CRM]) are likely to largely account for this positive mortality trend.
However, the management of locally advanced rectal cancer remains challenging in most cases. Indeed, despite the combined use of the available treatment options, a significant number of patients experience tumor progression and ultimately die of this disease. Moreover, although cure has historically represented the main objective of treatment, an increasing awareness of the potential treatment-related risks has recently led oncologists to evaluate alternative treatment strategies that may minimize toxicities and preserve quality of life, while maintaining a satisfactory oncological outcome.
In many countries in the USA and Europe, neoadjuvant long-course chemoradiotherapy or short-course radiotherapy followed by TME surgery are standard treatment options for patients with extramural tumor spread and/or nodal involvement [2–5]. Using such multimodality strategies reduces local recurrence rates to less than 10%. However, optimal quality TME without radiotherapy has been shown to be associated with similar rates of local recurrence [6,7]. Moreover, with the exception of a clinical trial and a meta-analysis (both conducted in the pre-TME era) [8–10], the improvement in local tumor control achieved with preoperative radiotherapy has not translated into a survival benefit [4–7].
As a result of the optimization of local tumor control, systemic tumor failure has now become the main challenge in locally advanced rectal cancer, occurring in 25–30% of patients and representing the cause of death in most cases [5,7]. Disappointingly, intensified radiosensitizing treatment strategies have largely failed to reduce the risk of distant metastases and improve the survival plateau for this disease [11–15]. In this scenario, it is legitimate to reconsider the role of radiotherapy and question whether adding radiotherapy to optimal quality TME is still beneficial for all patients or represents an unnecessary overtreatment for some patients. Indeed, radiotherapy is not without toxicities and may negatively impact on quality of life [16–21].
Neoadjuvant systemic chemotherapy without radiotherapy has emerged as an attractive therapeutic option to minimize the risk of treatment-related toxicities and potentially improve survival outcomes of patients with resectable, locally advanced rectal cancer. In this article, we describe the rationale and potential advantages of omitting radiotherapy in favor of systemic chemotherapy. We also review the available evidence on neoadjuvant chemotherapy without radiotherapy and anticipate a potential role for this investigational strategy within the context of a risk-adapted treatment paradigm.
Relative & absolute local recurrence risk reduction with radiotherapy
Radiotherapy has historically represented an essential component of the multimodality treatment of locally advanced rectal cancer [22]. The use of preoperative radiotherapy was largely based on the natural course of this disease that is characterized by high rates of local failure following curative surgery alone [23]. Moreover, the management of tumor recurrence within the pelvic or perineal area is challenging in most cases and this condition is frequently associated with pain, other disabling symptoms and poor quality of life [24,25].
Following conventional surgery, in which a blunt dissection of the rectal fascia was associated with a significant risk of suboptimal tumor resection and persistent microscopic disease, the incidence of local recurrence in resectable rectal cancer patients was reported to range from 15 to 45% [9,26–28]. Several randomized trials conducted in the 1980s and 1990s demonstrated a reduction in the rates of local recurrence with radiotherapy when given either before or after conventional surgery [29–37]. In the Swedish Rectal Cancer Trial [8], the largest of these randomized controlled trials (n = 1168), the cumulative proportion of local recurrence was 26% in the surgery alone group and 9% in the group of patients who received 5 × 5 Gy of preoperative radiotherapy (p < 0.001) [9]. After a median follow-up of 13 years, this trial also demonstrated that preoperative radiotherapy resulted in significant improvements in overall survival (38 vs 30%; p = 0.008) and cancer-specific survival (72 vs 62%; p = 0.03) [9].
Although associated with a magnitude of risk reduction similar to that observed in the era of conventional surgery, delivery of radiotherapy before TME (in which the entire mesorectum is enveloped and resected by precise, sharp dissection) [38,39] was shown to confer a lower absolute improvement in local tumor control. In the Dutch Colorectal Cancer Group trial [6], the largest randomized controlled trial conducted in the TME era (n = 1861), the 10-year cumulative incidence of local recurrence was 11% in the surgery-alone group and 5% in the radiotherapy group (5 × 5 Gy; p < 0.0001) [7]. In addition, in the subgroup of patients with a negative CRM the 10-year cumulative incidence of local recurrence was 9% in the surgery alone group and 3% in the radiotherapy group (p < 0.0001).
Overall, these figures suggest that, although still able to significantly improve the local tumor control, the impact of pre-operative radiotherapy in resectable rectal cancer is marginal when high-quality rectal surgery and accurate pathological assessment are performed. More importantly, although the primary end point of the Dutch Colorectal Cancer Group trial was local recurrence, this study clearly showed that the reduction in local recurrence achieved with radiotherapy did not translate into a survival advantage [6,7]. In a recent updated survival analysis of this trial, 10-year overall survival probabilities of 48 and 49% (p = 0.86) were reported for patients receiving preoperative radiotherapy and patients undergoing surgery alone, respectively. Likewise, no differences were observed between the two treatment arms with respect to cancer-specific survival (72 and 69% at 10 years; p = 0.20) with the exception of the subgroup of patients who had no CRM involvement after surgery (83 and 78%, respectively; p = 0.04) [7].
Although no randomized trials in the TME era have compared long-course preoperative chemoradiotherapy versus surgery alone, it is legitimate to assume that the impact of long course chemoradiotherapy on local tumor control and overall survival is similar to that reported for short-course radiotherapy. In the German CAO/ARO/AIO-94 trial, which demonstrated that local tumor control and tolerability of chemoradiotherapy was better when this treatment was given before than after TME, the cumulative incidence of local recurrence at 10 years in the intention-to-treat population was 7.1%, in line with the results of the Dutch trial [4,5]. Moreover, two randomized controlled trial comparing short-course radiotherapy and long-course chemoradiotherapy have reported similar outcomes with these two regimens. In the Polish trial, no differences were observed for all the study end points between short-course radiotherapy and chemoradiotherapy with bolus 5-fluorouracil (actuarial 4-year cumulative incidence of local recurrence 10.6 vs 15.6%, respectively; p = 0.21) [2,40]. These results have been recently confirmed by the Trans-Tasman Radiation Oncology Group Trial 01.04, which demonstrated no differences in the 5-year rates of local recurrence (7.5 vs 5.7%), distant recurrence (27 vs 30%) and overall survival (74 vs 70%) between short-course radiotherapy and long-course chemoradiotherapy with infusional 5-fluorouracil [41].
Radiotherapy-related toxicities
It has been historically acknowledged that using radiotherapy in rectal cancer increases the risk of acute, treatment-related toxicities. Although it is now clear that delivering radiotherapy before surgery is better tolerated compared with postoperative radiotherapy and does not significantly increase the risk of intraoperative complications or surgical reinterventions, a substantial proportion of patients treated with neoadjuvant radiotherapy develop, amongst others, gastrointestinal, dermatologic and neurologic toxicities [4]. Moreover, an increased frequency of postoperative complications, in particular perineal wound healing complications for patients undergoing abdominoperineal resection, and an increased number of early, postoperative readmission mainly for gastrointestinal diagnoses have been reported in trials of neoadjuvant radiotherapy [3,21,42].
Over the last two decades, especially following the overall improvement in overall survival of patients curatively treated for locally advanced rectal cancer, there has been an increased awareness of the potential mid- and long-term side effects of pelvic radiotherapy including bowel and urogenital disfunction and risk of second cancers. In the TME trial, long-term bowel dysfunction was reported to occur more frequently in irradiated patients than patients undergoing TME alone, with an increase in fecal incontinence, anal blood and mucus loss, and an overall higher impact of bowel dysfunction on daily activities [19]. The association between radiotherapy and long-term impairment of anorectal function has been recently confirmed in a systematic review and meta-analysis of 25 studies with 6548 patients [43].
A larger decline in sexual activity and deterioration of sexual functioning both for males and females who received preoperative short-course radiotherapy in the Swedish Rectal Cancer Trial was demonstrated and this effect did not appear to differ according to the type of surgery performed (low anterior resection versus abdominoperineal resection) [18]. Finally, pelvic radiotherapy has been reported to significantly increase the risk of second cancers amongst patients in follow-up for a curatively treated rectal cancer. In a pooled analysis of the Swedish Rectal Cancer Trial [8] and Uppsala Trial [44], the increased risk of second cancers (relative risk: 1.85) was mainly driven by tumors arising from organs within or adjacent to the irradiated volume (relative risk: 2.04) [20]. However, it is worth noting that the association between pelvic radiotherapy and increased risk of second cancers has not been confirmed in other series, possibly due to the balancing effect of a radiotherapy-related, inhibitory effect on spontaneously occurring tumors including prostatic adenocarcinomas [45].
Given the established acute and long-term adverse effects of rectal surgery [46–48] and the progressively increasing life expectancy, the additional morbidity potentially deriving from the use of pelvic radiotherapy should be taken into consideration when planning the treatment of patients with curable disease. In the next section, we will discuss if and how an individualized therapeutic approach in which predicted benefits of radiotherapy are carefully balanced against potential toxicities can be implemented in patients with locally advanced rectal cancer.
A selective approach to rectal cancer is feasible
The emerging concept of ‘stratified’ or ‘personalized medicine’ implies that a number of variables including clinical, pathological or molecular features of the tumors may contribute to the definition of tumor biological aggressiveness, influence the natural course of disease and dictate the patient individual prognosis. More importantly, identification at baseline of such variables also has clinical relevance in that clinicians can tailor specific anticancer treatments according to the patient individual risk profile. In this regard, although prognostic or predictive tumor biomarkers that impact on the routine management of locally advanced rectal cancer are still lacking [49], advances towards the implementation of a stratified approach for this disease have been recently made thanks to the increased ability of the pretreatment diagnostic imaging to accurately stage rectal cancers and predict their histopathologic features [50–55].
Current guidelines recommend that patients with T3–4 or N+ rectal cancers should be considered for a multimodality treatment approach including pre-operative radiotherapy [56,57]. The choice of neoadjuvant therapy (short-course radiotherapy versus long-course chemoradiotherapy) is based on the assessment of baseline clinico-pathologic risk factors including tumor site, depth of intramural penetration, involvement of the CRM and lymph node involvement. As previously mentioned, two clinical trials showed similar outcome results for patients treated with short-course radiotherapy or long-course chemoradiothereapy [40,41]. However, long-course chemoradiotherapy is generally the preferred treatment approach in low-lying tumors or in situations where the CRM is predicted to be involved/at risk and significant tumor downstaging/downsizing is necessary.
A tailored approach to rectal cancer has been demonstrated to be feasible and not associated with a negative impact on the overall oncological outcome. In The Netherlands, the use of a differentiated treatment strategy based on the location of the tumor, CRM involvement and N status (as identified on baseline MRI), has been recently evaluated in a prospective, multicenter, cohort study (n = 230) [58]. High-risk tumors (CRM ≤2 mm, N2 tumors and T3 and/or N1 tumors in the distal rectum) were treated with long-course chemoradiotherapy, low-risk tumors (T1–2, N0 or T3N0 proximal rectal cancers) were treated with TME surgery alone, and short-course radiotherapy was administered to all the other, so-called, intermediate-risk tumors. Although this study did not include a control group of uniformly treated patients, the results of this tailored strategy (local recurrence, disease-free survival and overall survival at 3 years: 2.2, 80.2 and 84.5%, respectively) appeared to compare favorably with those of randomized prospective series where baseline MRI and/or a MRI-based selective treatment was not routinely performed. Similar results were previously reported by the investigators of the prospective, multicenter, MERCURY study [59]. Although this study did not include a predefined, risk-adapted, treatment algorithm and the individual therapeutic strategies were chosen according to the local institution policies, the subgroup of 122 patients who had not received any preoperative treatment based on the good tumor prognostic features indicated by the baseline MRI was found to have a 5-year local relapse of 3.3%, 5-year disease-free survival of 84.7% and 5-year overall survival of 68.2%. It is interesting to note that, in contrast with the study conducted in The Netherlands, the good prognosis group in the MERCURY study also included patients with extramural spread <5 mm (T3a and T3b) regardless of the tumor location and patients with any N stage. This outlines the existing controversy in evaluating the risk associated with specific tumor characteristics and the absence of a universally accepted definition of locally advanced rectal cancer. In this regard, the different risk profile associated with tumor stage and other clinico-pathological characteristics was the basis for an alternative risk classification of rectal cancer, which reflected the adoption of a routine risk-adapted treatment algorithm in Sweden. Of note, this algorithm primarily addressed the risk of local failure and classified tumors in three groups according to the local recurrence rates reported after surgery alone: favorable ‘good’ group (T1–3b mid/upper tumors, T1–T3a low tumors, N0) intermediate ‘bad’ group (T3c/d mid/upper tumors, T3b low tumors, T4 tumors due to peritoneal or vaginal involvement, N+) and advanced ‘ugly’ group (all the other T4 tumors and tumors with CRM involvement [60].
Altogether, these data suggest that not all rectal cancer patients need preoperative radiotherapy and a more individualized treatment approach, tailored on the individual risk factors at baseline, is certainly feasible, is associated with an excellent oncological outcome and may also potentially reduce the risk of unnecessary overtreatment.
• Why neoadjuvant chemotherapy without radiotherapy may be an option: potential advantages
The survival plateau of patients with locally advanced disease and the data on the late adverse effects of pelvic radiotherapy legitimate the question as to whether systemic chemotherapy without radiotherapy may improve the outcome of these patients (especially by reducing the risk of distant metastases) or at least offer similar outcome (especially in term of risk of local tumor recurrence) with less toxicity and better quality of life. Although there are currently no available data from randomized controlled studies comparing these two treatment strategies, several theoretical points can be considered to evaluate what potential advantages may be obtained with the administration of preoperative systemic chemotherapy without radiotherapy.
Although the role of adjuvant chemotherapy in rectal cancer patients who had previously received preoperative radiotherapy is still controversial [61–65], administering a course of 4–6 months of a fluoropyrimidine-based systemic chemotherapy to (high-risk) stage II and stage III patients is a common practice. However, it is established that, following radiotherapy and rectal surgery, the rates of adherence to adjuvant chemotherapy is low with only approximately 75% of patients starting chemotherapy and 42–57% receiving the planned dose of treatment [66,67]. Moreover, adjuvant chemotherapy after rectal surgery is associated with an increased risk of toxicity and worse tolerability compared with when the same chemotherapy regimen is delivered preoperatively [67]. Finally, the common practice of selecting patients for adjuvant chemotherapy based on the clinical stage indicated by the preoperative imaging (rather than on the postoperative histopathologic findings) could potentially lead to an overtreatment in some cases [4]. Indeed, although endoscopic ultrasound and MRI are both relatively accurate in the preoperative assessment of T, the evaluation of the lymph node involvement still remains a challenge [68–70].
Much of the information on the use of neoadjuvant chemotherapy has been made available by the results of clinical trials of intensified treatment strategies in which neoadjuvant systemic chemotherapy was administered prior to chemoradiotherapy in locally advanced rectal cancer [15,67,71]. In these trials, delivery of combination chemotherapy at full systemic doses was shown to be feasible and associated with good tolerability and high compliance. High response rates to systemic chemotherapy (54–74%) and significant tumor shrinkage were reported, and the risk of tumor progression while on treatment was negligible (<3%). Moreover, the use of upfront systemic chemotherapy did not appear to reduce the therapeutic potential of preoperative chemoradiotherapy and did not adversely affect the rate of R0 resection. Finally, even in the context of a more intensified treatment regimen including chemoradiotherapy, the use of preoperative systemic chemotherapy did not significantly increase the risk of postoperative morbidity and mortality when compared with standard treatment.
It is also legitimate to hypothesise that using neoadjuvant chemotherapy without radiotherapy has further potential advantages which may ultimately improve the survival outcomes of patients with locally advanced disease. The early use of combination chemotherapy at full systemic doses may result in an increased therapeutic effect on potential foci of micrometastatic disease and reduce the risk of distant failure, the major cause of cancer-related death in rectal cancer. Tumor response to preoperative chemotherapy can be used as a surrogate of in vivo chemotherapy sensitivity and may ultimately provide a more reliable tool to guide treatment selection in the postoperative setting than baseline clinical staging. The high rates of tumor response to systemic chemotherapy observed in metastatic colorectar cancer and in previous trials of induction chemotherapy in rectal cancer suggest that chemotherapy may potentially be more effective than chemoradiotherapy in downstaging/downsizing rectal cancers which involve the mesorectal fascia (CRM positive) or invade adjacent organs (T4; the so called ‘ugly tumors’ of the aforementioned Swedish classification) [60]. This is especially true considering the potential of increasing the antitumor activity of systemic chemotherapy with the addition of targeted agents including anti-EGF receptor monoclonal antibodies (cetuximab or panitumumab) in RAS wild-type patients or anti-angiogenic drugs (bevacizumab or aflibercept) in unselected populations [72–74]. Patients with T4 or CRM-positive tumors have been excluded from most studies of neoadjuvant chemotherapy without radiotherapy (as reported in the next sections), and data on the potential of systemic chemotherapy alone to downsize these tumors are lacking. However, it is worth noting that, in the EXPERT-C trial, the response rate (according to RECIST [Response Evaluation Criteria In Solid Tumors] criteria) observed after four cycles of neoadjuvant chemotherapy plus cetuximab in patients with RAS wild-type tumors (as assessed by MRI scan performed before sequential chemoradiotherapy) was 78% [75]. More interestingly, in this patient population, T4 tumors and tumors with CRM involvement were present in 24 and 55% of cases, respectively. However, ad hoc studies are needed to address whether replacing radiotherapy with systemic chemotherapy in patients with high-risk features is associated with a rate of local recurrence that is comparable to that observed with standard preoperative chemoradiotherapy.
• When neoadjuvant chemotherapy without radiotherapy may be an option: a hypothetical therapeutic algorithm
Although neoadjuvant systemic chemotherapy without radiotherapy is an attractive treatment option for patients with locally advanced rectal cancer, it is not clear which patient or tumor characteristics should be used to select patients for this alternative strategy. Moreover, it is crucial to understand whether omitting radiotherapy in favor of systemic chemotherapy is safe in terms of risk of local recurrence especially for distal tumors that need an abdomino-perineal resection, tumors invading adjacent organs or tumors with predicted CRM involvement. There are very limited data currently available on neoadjuvant chemotherapy without radiotherapy in rectal cancer and prospective clinical trials assessing this treatment strategy are ongoing (these will be discussed in the next sections). However, it could be hypothesized that systemic chemotherapy alone may be an appropriate treatment for any patients who would require preoperative chemoradiotherapy to downstage/downsize the tumor and achieve a safe CRM or any patient who is predicted to be a candidate for adjuvant combination chemotherapy (lymph nodal involvement, good performance status and age ≤70 years). Indeed, factors such as CRM involvement, advanced T (≥T3c) and N+ disease are associated with an increased risk of distant failure, which is the most important prognostic determinant in these patients [76–78]. The low rates of local recurrence postoptimal quality TME surgery have minimized the role of preoperative radiotherapy and, even in the presence of low lying tumors, bulky disease or CRM involvement, the impact of systemic failure on survival outcome by far exceeds the potential risk associated with the use of a strategy, which may provide a suboptimal local tumor control [77,79]. Neoadjuvant chemotherapy without radiotherapy could also be a potential option for those patients whose tumors are predicted to invade the veins beyond the muscolaris propria (extramural venous invasion), which has been suggested to be a poor prognostic factor in resectable rectal cancer due to its association with distant metastases [80].
It is important to note that the use of selective treatment strategies for patients with locally advanced rectal cancer is largely reliant on the ability of the currently available imaging techniques to accurately stage rectal tumors and to identify high-risk features at baseline. In this regard, MRI has been demonstrated to provide an accurate assessment of the depth of tumor extramural invasion, tumor distance from the mesorectal fascia and invasion of the extramural veins [81–83]. However, despite the recent improvements associated with the use of new diagnostic criteria and more sensitive techniques, the accuracy of MRI in assessing lymph node involvement is still suboptimal [84]. False-positive results could potentially result in overtreatment of patients whose tumors do not present additional high-risk features and would be otherwise candidate for upfront surgical resection.
In contrast, the ability of MRI to accurately predict tumor response [85] to treatment could offer a useful tool to implement an adaptive treatment strategy where patients who do not respond to upfront systemic chemotherapy or have insufficient tumor downsizing and are still at risk of CRM involvement are switched to preoperative chemoradiotherapy (Figure 1).
Available data on neoadjuvant chemotherapy without radiotherapy
Despite the available data suggesting a controversial effect of radiotherapy on survival outcome and the rationale supporting a potential role for an approach with preoperative systemic chemotherapy alone, very few studies have been conducted so far to assess neoadjuvant chemotherapy without radiotherapy in locally advanced rectal cancer (Table 1).
In 2010, Ishii et al. reported the medium-term results of a prospective study with two cycles of neoadjuvant sytemic chemotherapy with IFL (irinotecan, bolus 5-FU and leucovorin) in 26 patients with T3–4, any N rectal cancers located within 12 cm of the anal verge (mid and lower rectum) [86]. All the patients were carefully staged with endoscopy, CT scan (chest, abdomen and pelvis) and MRI pelvis before and after chemotherapy and underwent TME with bilateral pelvic lymphadenectomy (if the tumor was located below the peritoneal reflection) 2–4 weeks after the completion of neoadjuvant therapy. Patients with lymph node involvement underwent postoperative adjuvant chemotherapy with oral fluoropyrimidines. Tumor downstaging (T and/or N) occurred in 15 patients (57.7%), R0 resection was performed in all the patients, and pathologic complete response was observed in one case (3.8%). Postoperative complications including anastomotic leakage and wound infection occurred in four patients (15.4%). After a median follow-up of 75 months, three patients (11.5%) had local recurrence while two (7.7%) experienced distant metastases. The authors reported a 5-year relapse-free survival and overall survival rates of 74 and 84%, respectively. Of note, this study included only three (12%) patients with T4 tumors whereas the number of patients with CRM involvement was not reported.
Investigators from the Memorial Sloan–Kettering Cancer Center (NY, USA) and the Dana-Farber Cancer Institute (MA, USA) reported a small retrospective series of patients who received preoperative FOLFOX (bolus 5-fluorouracil, infusional 5-fluorouracil, folinic acid and oxaliplatin) without radiotherapy as initial management of locally advanced rectal cancer, either because they had suspected metastatic disease, or relative contraindications to radiotherapy or refused radiotherapy [87]. Of the six patients who received this treatment for localized disease (T2–3, N1), two were found to have a pathologic complete response and three had a significant tumor downstaging. Although the small sample size and the absence of ‘ugly’ prognostic features preclude any definitive interpretation of these results, the authors reported that all the patients with significant tumor response were free of disease at time of the last follow-up while the patient with minimal treatment effect in the primary tumor experienced distant metastases without local recurrence.
The use of a targeted agent in combination with neoadjuvant chemotherapy without radiotherapy has been recently tested in a Japanese single-arm Phase II study [89]. MRI-defined, high-risk rectal cancer patients (n = 32) received four cycles of CAPOX (capecitabine and oxaliplatin) plus bevacizumab (the latter was not administered during the fourth cycle of therapy) followed by TME with lateral pelvic lymph nodal dissection for tumor located at or below the peritoneal reflection. Adjuvant chemotherapy was not included in the study protocol. The study included 19 (52%) patients with T4 tumors (T4a in nine cases and T4b in ten cases) and 12 (38%) with N2 disease. Although CRM involvement was one of the high-risk features included in the study eligibility criteria, the number of patients with CRM-positive tumors was not reported. Response to treatment was observed in 62.5% of patients whereas progressive disease before surgery was reported in one case. A total of 29 patients completed the neoadjuvant treatment and 27 (84.3%) underwent an R0 resection. Pathologic complete response was reported in four out of 32 patients (12.5%) and good tumor regression was reported in 11 cases (34.4%). Interestingly, 20 out of 24 (83.3%) patients with lymph node involvement at baseline were found to have pN0 disease at the time of pathological assessment. Although the neoadjuvant treatment was overall well tolerated, it is worth noting that one patient (3.1%) died due to rectal perforation and 13 out of 30 patients (43%) who underwent resection developed some complications including wound sepsis (seven patients [23.3%]), pelvic sepsis (three patients [10%]) and anastomotic leakage (five out of 18 patients treated with sphyncter-preserving surgery [27.8%]).
Although these results question the safety of bevacizumab in the neoadjuvant setting before rectal surgery, they are in contrast with the results of a pilot prospective study assessing the feasibility of achieving R0 resection with neoadjuvant FOLFOX plus bevacizumab administered without radiotherapy in intermediate risk, locally advanced rectal cancer (uT2–3, any N except bulky N2) [90]. Patients with T4 tumors, tumors with CRM involved/at risk or unresectable disease were not included. In this study, six cycles of systemic chemotherapy (bevacizumab administered only with the first four cycles) were followed by TME if tumor response or stable disease was observed, otherwise subsequent 5-fluorouracil-based chemoradiotherapy was considered. Although the postoperative management was at the discretion of the treating physician, six further cycles of FOLFOX were recommended. In total, 30 patients completed the planned neoadjuvant treatment and in only two cases, systemic chemotherapy was replaced by chemoradiotherapy as a result of toxicity. All patients had an R0 resection and pathologic complete response was achieved in eight cases (25%). Moreover, in 15 out of 32 (46.9%) patients a tumor regression >50% was observed. Only one postoperative death was reported (3.1%) and attributed to renal failure following high volume ileostomy output. After a median follow-up of 54 months, no local recurrences were observed and only four patients (12.5%) experienced a distant failure. Although limited information is available on the incidence of perioperative complications, it is possible that the overall good safety of neoadjuvant bevacizumab in this study, as opposed to the Japanese study, is related to the longer interval between the last dose of bevacizumab and surgery and the use of a temporary diverting oostomy in most cases.
Further preliminary data on the use of neoadjuvant chemotherapy plus bevacizumab without radiotherapy is available on 28 patients enrolled into the GEMCAD 0801 study [88]. In this single-arm Phase II trial, patients with, T3 tumors of the mid-rectum (T4 tumors and tumors with CRM involvement excluded) received four cycles of CAPOX plus bevacizumab (last cycle without bevacizumab). Only three out of 27 (11.1%) patients experienced grade ≥3 surgical complications; however, one patient (3.6%) died of perforation during chemotherapy. Similarly to the above mentioned study, good response to treatment was observed and promising pathologic complete response rate (15%) and R0 resection rate (96%) were reported.
There is no doubt that the general applicability of these results is limited by the small number of patients analyzed, the absence of established, risk-adapted criteria for patient selection, the exclusion of ‘ugly tumors’ in most cases, the absence of a control group treated with preoperative radiotherapy and the short follow-up. Moreover, the inter- and intra-study patient heterogeneity do not allow a definitive interpretation of the role of neoadjuvant chemotherapy with the promising treatment outcomes reported in these study being potentially associated with the selection of patients with favorable tumor features at baseline. However, these data suggest that selected patients with rectal cancer may be treated with upfront systemic chemotherapy and spared the toxic effects of pelvic radiotherapy without adversely affecting R0 resection rates, pathologic complete response rates and possibly overall survival outcomes.
Ongoing trials of neoadjuvant chemotherapy without radiotherapy
Based on the promising preliminary results of previous studies, several clinical trials are currently investigating neoadjuvant systemic chemotherapy without radiotherapy in patients with locally advanced rectal cancer.
FOWARC is a three-arm, randomized Phase II/III trial, sponsored by Sun Yat-sen University (China) (ClinicalTrials.gov identifier: NCT01211210) [91], which compares conventional chemoradiotherapy with FOLFOX chemotherapy plus radiotherapy or FOLFOX chemotherapy alone. Of note, eligibility in this trial is not restricted to a specific subgroup of patients but all the patients with stage II and III rectal cancer, regardless of the presence of high-risk features at baseline, are potentially eligible. The primary end point of this study is 3-year disease-free survival.
A cooperative group study sponsored by the North Central Cancer Treatment Group in collaboration with the National Cancer Institute (PROSPECT, NCCTC N1048; ClinicalTrials.gov identifier: NCT01515787) [92], is assessing neoadjuvant FOLFOX, with selective use of chemoradiotherapy versus conventional preoperative chemoradiotherapy for patients candidate for low anterior resection for intermediate-risk rectal cancer (clinical stage T2N1, T3N0 or T3N1). In this randomized Phase II/III trial, patients with less than 20% of tumor regression after neoadjuvant chemotherapy undergo chemoradiotherapy as in the control group before proceeding to surgery. The primary endpoint. of the Phase II component is the R0 resection rate, while co-primary end points of the Phase III component are disease-free survival and time to local recurrence.
The BACCHUS trial, a randomized Phase II study sponsored by University College London (London, UK; ClinicalTrials.gov identifier: NCT01650428) [93], is evaluating the safety, feasibility and efficacy of two combination chemotherapy regimens in the neoadjuvant setting. In this trial, patients with poor prognosis tumor features as defined at baseline MRI (T3b, T3c or T3d, any N or presence of macroscopic extramural venous invasion) but with no CRM threatened/involved are randomized to FOLFOX plus bevacizumab or FOLFOXIRI plus bevacizumab (six cycles, bevacizumab is omitted from the final cycle) without radiotherapy. Interestingly, only patients who have a metabolic response (≥30% decrease in the standardized uptake value compared with baseline PET/computed tomography) after three cycles of chemotherapy are eligible to receive three further cycles of treatment within the study. The primary end point of this trial is pathologic complete response.
Conclusion
Multimodality treatment including pelvic radiotherapy has been considered for years the standard approach to patients with locally advanced rectal cancer. Preoperative (chemo)radiotherapy followed by surgery and adjuvant chemotherapy still represents the treatment of choice in most cases. However, it has been increasingly recognized that a critical re-evaluation of this approach is required and more selective treatment strategies should be implemented which may offer a better balance between expected individual clinical benefits and treatment-related toxicities.
The improvement in local tumor control achieved with the widespread use of high-quality surgery and the standardization of the criteria for the pathological assessment of resection specimens have minimized the impact of pelvic radiotherapy on both local recurrence and overall survival. Results from clinical trials conducted in the TME era consistently suggest that, at least in some cases, radiotherapy may not be a necessary component of the multimodality treatment for locally advanced rectal cancer. Preliminary prospective data confirm the hypothesis that omitting radiotherapy may not adversely affect the oncological outcome of these patients (also in terms of local recurrence); however, it might have the advantage of reducing acute and late treatment-related toxicities and preserving quality of life [94]. Moreover, there is hope that replacing preoperative (chemo)radiotherapy with full-dose systemic chemotherapy could significantly reduce the incidence of distant metastases and ultimately improve the survival of patients curatively treated patients.
Although a selective approach to locally advanced rectal cancer has been shown to be feasible, randomized prospective evidence (including data on survival outcomes, local tumor control, toxicity and quality of life) is necessary to conclude that neoadjuvant chemotherapy without radiotherapy may be a valuable, alternative therapeutic option. Moreover, the criteria to select patients for a treatment strategy without radiotherapy remain uncertain.
In conclusion, systemic chemotherapy without radiotherapy could be a new treatment approach to patients with locally advanced rectal cancer. However, until this strategy is confirmed to be at least noninferior compared with standard (chemo)radiotherapy, its use should be considered investigational or limited to those patients with contraindications to pelvic radiotherapy.
Future perspective
Given the high anti-tumor activity of systemic chemotherapy, any patients (including those with bulky tumors or tumors with high-risk features such as CRM involvement or distal location) could be theoretically deemed candidate for an approach in which radiotherapy is delivered preoperatively if a substantial tumor downsizing is not achieved after upfront systemic chemotherapy or postoperatively if the pathologic CRM is involved. The results of ongoing clinical trials investigating neoadjuvant systemic chemotherapy without radiotherapy in different patient populations will provide useful information on the efficacy of this treatment strategy and the most appropriate criteria to use for treatment selection. However, studies in patients with high-risk features for local recurrence are necessary to understand whether preoperative pelvic radiotherapy can be safely omitted without significantly affecting the chances of radical resection and local tumor control. Moreover, several questions remain unanswered and need to be addressed before supporting the general applicability of a hypothetical treatment algorithm without preoperative radiotherapy.
Despite recent advances in diagnostic imaging have led to an increased accuracy in staging rectal cancer, an improvement in sensitivity and specificity for the detection of lymph node metastases at baseline is necessary to minimize the risk of overtreatment due to false-positive results. Indeed, although better tolerated than adjuvant chemotherapy, neoadjuvant chemotherapy may be poorly accepted by patients and associated with disabling symptoms and potentially life-threatening toxicities.
Based on the ability to assess treatment response and predict pathological findings, MRI is a valuable tool for the identification of those patients who may or may not benefit from the use of additional preoperative radiotherapy. However, studies are necessary to investigate and validate imaging parameters, which may reliably assess the efficacy of neoadjuvant chemotherapy. Changes in tumor size and metabolic activity are currently used in the aforementioned ongoing clinical trials to guide treatment decision after neoadjuvant chemotherapy and to make recommendation on the use of sequential preoperative chemoradiotherapy. However, other parameters including CRM involvement, T or N downstaging and MRI tumor regression grade may potentially offer a better estimate of the biological effects of neoadjuvant systemic chemotherapy and warrant investigation in this setting.
Validation of algorithms of treatment selection based on the accurate assessment of the individual patient risk profile and the potential effect on both oncologic outcome and safety/quality of life of a given treatment strategy is needed. Treatment selection in locally advanced rectal cancer is still based on clinical risk stratification but does not take into account the multifaceted biology of these tumors. There are currently no tumor biomarkers which have been shown to predict response to radiotherapy or chemotherapy, specific pattern of tumor relapse and overall prognosis. Identification and validation of reliable and reproducible biomarkers, and implementation of biomarker-driven, selective strategies for patients with locally advanced rectal cancer should be the focus of future research.
Finally, evaluation of patients’ quality of life and long-term functional outcome should be key endpoints to incorporate in clinical trials investigating alternative neoadjuvant treatment strategies for rectal cancer.
| Study (year) | Patients (n) | Patient population | Patients with ‘ugly’ features† | Treatment | Follow-up (months) | R0 resection rate (%) | pCR (%) | Local relapse (%) | Distant relapse (%) | Relapse-free survival (%) | Overall survival (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ishii et al. (2010) | 26 | T3–4, any N, within 12 cm of the anal verge | T4: 3/26 (12%) CRM+: NR | IFL | 75 | 100 | 3.8 | 11.5 | 7.7 | 74‡ | 84‡ | [86] |
| Cercek et al. (2010) | 6 | T2–3, N1 | T4: 0% CRM+: NR | FOLFOX | NA | 100 | 33.3 | 0 | 16.7 | NA | NA | [87] |
| Fernandez-Martos et al. (2012) | 28 | T3 middle third tumors ≥2 mm from the mesorectal fascia | T4: 0% CRM+: 0% | CAPOX – bevacizumab | NA | 96.4 | 14.3 | NA | NA | NA | NA | [88] |
| Uehara et al. (2013) | 32 | T3 >5 mm, T4, N2, CRM involved/at risk | T4a: 9/32 (28%) T4b: 10/32 (31%) CRM+: NR | CAPOX – bevacizumab | NA | 84.3 | 12.5 | NA | NA | NA | NA | [89] |
| Schrag et al. (2014) | 32 | T2N1, T3 any N (except N2 bulky), within 5 and 12 cm of the anal verge | T4: 0% CRM+: 0% | FOLFOX – bevacizumab | 54 | 100 | 25.0 | 0 | 12.5 | 92§ | 91.6§ | [90] |
EXECUTIVE SUMMARY
Background
• Neoadjuvant long-course chemoradiotherapy or short-course radiotherapy followed by total mesorectal excision is a standard of care for patients with locally advanced rectal cancer.
• Alternative therapeutic options are under investigation to reduce the risk of treatment-related toxicities and improve the outcome.
Relative & absolute local recurrence risk reduction with radiotherapy
• Before the standardization of total mesorectal excision, delivery of preoperative radiotherapy was associated with a reduction in local recurrence and an improvement in survival.
• If high-quality rectal surgery and optimal pathological assessment are performed, the impact of preoperative radiotherapy on local tumor control is marginal and does not translate into a survival benefit.
Radiotherapy-related toxicities
• Preoperative pelvic radiotherapy is associated with acute, mid- and long-term side effects including bowel and urogenital disfunction and risk of second cancers.
A selective approach to rectal cancer is feasible
• A tailored approach to rectal cancer is feasible and does not compromise the overall oncological outcome.
• Following the improvements in diagnostic imaging, it is possible to identify those patients who may not benefit from the use of preoperative radiotherapy.
Why & when neoadjuvant chemotherapy without radiotherapy may be an option
• Upfront combination chemotherapy without radiotherapy yields several theoretical advantages including delivery of chemotherapy at full systemic doses, early treatment of micrometastases and reduction of the risk of distant failure.
• Neoadjuvant chemotherapy without radiotherapy may be an option for patients who need tumor downstaging/downsizing to achieve a safe circumferential resection margin or patients who are likely to receive adjuvant combination chemotherapy.
Available data on neoadjuvant chemotherapy without radiotherapy
• Very few studies of neoadjuvant chemotherapy without radiotherapy have been conducted.
• Overall, the available data suggest that this treatment strategy is associated with promising short- and mid-term outcomes.
Ongoing trials of neoadjuvant chemotherapy without radiotherapy
• Prospective clinical trials are currently investigating the role of neoadjuvant chemotherapy without radiotherapy in locally advanced rectal cancer.
Conclusion
• Systemic chemotherapy without radiotherapy is a promising treatment strategy for patients with locally advanced rectal cancer. More data are needed before this approach may become a new standard of care.
Future perspective
• Characterization of the individual patient risk profile and prediction of treatment response by means of reliable and reproducible tumor biomarkers are crucial to identify those patients who are most likely to benefit from the use of neoadjuvant chemotherapy without radiotherapy.
Financial & competing interests disclosure
The authors acknowledge support from the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research. We also acknowledge funding from the Peter Stebbings Memorial Charity. D Cunningham has received research funding from Roche, Amgen, Celgene, Sanofi, Merck Serono, Novartis, AstraZeneca, Bayer, Merrimack and MedImmune. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1 Cancer Research UK. www.cancerresearchuk.org/cancer-info/cancerstats/.
- 2 . Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 93(10), 1215–1223 (2006).
- 3 Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 373(9666), 811–820 (2009).
- 4 Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 351(17), 1731–1740 (2004).
- 5 Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized Phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 30(16), 1926–1933 (2012).
- 6 Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 345(9), 638–646 (2001).
- 7 Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 12(6), 575–582 (2011).•• Study demonstrating that, if high-quality surgery is performed, the reduced risk of local recurrence with preoperative radiotherapy does not translate into a survival benefit.
- 8 Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N. Engl. J. Med. 336(14), 980–987 (1997).
- 9 . Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J. Clin. Oncol. 23(24), 5644–5650 (2005).
- 10 . Preoperative rdiotherapy for resectable rectal cancer: a meta-analysis. JAMA 284(8), 1008–1015 (2000).
- 11 Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized Phase III trial. J. Clin. Oncol. 29(20), 2773–2780 (2011).
- 12 Neoadjuvant therapy for rectal cancer: mature results from NSABP protocol R-04. J. Clin. Oncol. 32(Suppl. 3), Abstract 390 (2014).
- 13 Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised Phase 3 trial. Lancet Oncol. 13(7), 679–687 (2012).
- 14 Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J. Clin. Oncol. 30(36), 4558–4565 (2012).
- 15 Multicenter randomized Phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J. Clin. Oncol. 30(14), 1620–1627 (2012).
- 16 Late side effects and quality of life after radiotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 76(4), 1005–1011 (2010).
- 17 . Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: results from a randomized Phase III study. Int. J. Radiat. Oncol. Biol. Phys. 81(4), 1017–1024 (2011).
- 18 Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J. Clin. Oncol. 23(9), 1847–1858 (2005).• Study reporting the adverse effects of preoperative radiotherapy on sexual functioning.
- 19 Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients – a Dutch Colorectal Cancer Group Study. J. Clin. Oncol. 23(25), 6199–6206 (2005).• Study reporting the adverse effects of preoperative radiotherapy on bowel function.
- 20 . Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J. Clin. Oncol. 23(25), 6126–6131 (2005).• Study reporting an increased risk of second cancers following the use of preoperative radiotherapy for rectal cancer.
- 21 . Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J. Clin. Oncol. 23(24), 8697–8705 (2005).
- 22 Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N. Engl. J. Med. 312(23), 1465–1472 (1985).
- 23 Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Swedish Rectal Cancer Trial. Eur. J. Surg. 162(5), 397–402 (1996).
- 24 . Health-related quality of life after surgery for primary advanced rectal cancer and recurrent rectal cancer: a review. Colorectal Dis. 14(7), 797–803 (2012).
- 25 Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. The Beyond TME Collaborative. Br. J. Surg. 100(8), 1009–1014 (2013).
- 26 . Radical abdominopelvic lymphadenectomy: historic perspective and current role in the surgical management of rectal cancer. Dis. Colon Rectum 37(1), 73–87 (1994).
- 27 . Local recurrence following ‘curative’ surgery for large bowel cancer. I. The overall picture. Br. J. Surg. 71(1), 12–16 (1984).
- 28 Local recurrence in patients with rectal cancer, diagnosed between 1988 and 1992: a population-based study in the west Netherlands. Eur. J. Surg. Oncol. 24(6), 528–535 (1998).
- 29 Stockholm Colorectal Cancer Study Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann. Surg. Oncol. 3(5), 423–430 (1996).
- 30 Low-dose preoperative radiation postpones recurrences in operable rectal cancer. Results of a randomized multicenter trial in western Norway. Cancer. 66(11), 2286–2294 (1990).
- 31 . Long-term results of a randomised trial of short-course low-dose adjuvant pre-operative radiotherapy for rectal cancer: reduction in local treatment failure. Eur. J. Cancer 30A(11), 1602–1606 (1994).
- 32 . The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 75(9), 2269–2275 (1995).
- 33 Medical Research Council Rectal Cancer Working Party. Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 348(9042), 1605–1610 (1996).
- 34 Medical Research Council Rectal Cancer Working Party. Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Lancet 348(9042), 1610–1614 (1996).
- 35 . Adjuvant preoperative radiotherapy for locally advanced rectal carcinoma. Results of a prospective, randomized trial. Dis. Colon Rectum. 37(12), 1205–1214 (1994).
- 36 . Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother. Oncol. 13(4), 245–252 (1988).
- 37 Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J. Natl Cancer Inst. 80(1), 21–29 (1988).
- 38 . A new approach to rectal cancer. Br. J. Hosp. Med. 22(3), 277–281 (1979).
- 39 Effect of the plane of surgery achieved on local recurrence in patients operated with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTGCO16 randomised clinical trial. Lancet 373(9666), 821–828 (2009).
- 40 Sphincter preservation following preoperative radio-therapy for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother. Oncol. 72(1), 15–24 (2004).
- 41 Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J. Clin. Oncol. 30(31), 3827–3833 (2012).
- 42 Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J. Clin. Oncol. 20(3), 817–825 (2002).
- 43 Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann. Surg. Oncol. 20(6), 1816–1828 (2013).• Meta-analysis showing the long-term adverse effects of preoperative radiotherapy on anorectal function.
- 44 . Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis. Colon Rectum 36(6), 564–572 (1993).
- 45 . A population-based analysis of second primary cancers after irradiation for rectal cancer. Am J. Clin. Oncol. 30(4), 333–339 (2007).
- 46 Risk factors for faecal incontinence after rectal cancer treatment. Br. J. Surg. 94(10), 1278–1284 (2007).
- 47 . Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst. Rev. 12, CD004323 (2012).
- 48 . A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann. Surg. Oncol. 14(7), 2056–2068 (2007).
- 49 . Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 74(3), 673–688 (2009).
- 50 . Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 71(2), 456–461 (2008).
- 51 Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann. Surg. Oncol. 19(9), 2842–2852 (2012).
- 52 Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J. Clin. Oncol. 29(28), 3753–3760 (2011).
- 53 Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann. Surg. Oncol. 19(4), 1199–1205 (2012).
- 54 Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J. Clin. Oncol. 32(1), 34–43 (2014).
- 55 MRI-based indications for neoadjuvant radiochemotherapy in rectal carcinoma: interim results of a prospective multicenter observational study. Ann. Surg. Oncol. 18(10), 2790–2799 (2011).
- 56 . ESMO Guidelines Working Group. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24(Suppl. 6), vi81–vi8 (2013).
- 57 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Rectal Cancer Version 2.2014. www.NCCN.org
- 58 Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur. J. Cancer. 49(10), 2311–2320 (2013).•• Study demonstrating that a selective treatment approach to patients with rectal cancer is feasible.
- 59 Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann. Surg. 253(4), 711–719 (2011).•• Study demonstrating that preoperative radiotherapy may not be necessary in locally advanced rectal cancer patients with good prognosis features at baseline MRI.
- 60 . The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol. 47(1), 5–8 (2008).
- 61 Quasar Collaborative Group, . Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370(9604), 2020–2029 (2007).
- 62 . Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann. Oncol. 21(12), 1743–1750 (2010).
- 63 . Adjuvant chemotherapy in rectal cancer – an issue or a nonissue? Ann. Oncol. 21(9), 1739–1741 (2010).
- 64 . Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst. Rev. 3, CD004078 (2012).
- 65 The value of adjuvant chemotherapy in rectal cancer patients after preoperative radiotherapy or chemoradiation followed by TME-surgery: The PROCTOR/SCRIPT study. Presented at: 2013 European Cancer Congress, Amsterdam, The Netherlands, 27 September–1 October 2013 (Abstract 1).
- 66 Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 355(11), 1114–1123 (2006).
- 67 Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 study. J. Clin. Oncol. 28(5), 859–865 (2010).
- 68 . Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging – a meta-analysis. Radiology 232(3), 773–783 (2004).
- 69 . Optimal methods for staging rectal cancer. Clin. Cancer Res. 13(22 Pt 2), s6877–s6884 (2007).
- 70 Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann. Surg. Oncol. 19(7), 2212–2223 (2012).
- 71 Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a Phase 2 trial. Lancet Oncol. 11(3), 241–248 (2010).
- 72 Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 369(11), 1023–1034 (2013).
- 73 Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: a randomized Phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. 2013 European Cancer Congress, Amsterdam, The Netherlands, 27 September–1 October 2013 (Abstract LBA17).
- 74 FOLFOXIRI/bevacizumab (bev) versus FOLFIRI/bev as first-line treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): results of the Phase III TRIBE trial by GONO group. J. Clin. Oncol. 31(Suppl.), Abstract 3505 (2013).
- 75 RAS mutations and cetuximab in locally advanced rectal cancer: results of the EXPERT-C trial. Eur. J. Cancer. 50(8), 1430–1436 (2014).
- 76 . The prognostic inhomogeneity in pT3 rectal carcinomas. Int. J. Colorectal Dis. 16(5), 298–304 (2001).
- 77 . What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 26(2), 303–312 (2008).
- 78 Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J. Clin. Oncol. 29(23), 3163–3172 (2011).
- 79 . Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis. Colon Rectum. 47(1), 48–58 (2004).
- 80 EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann. Oncol. 25(4), 858–863 (2014).
- 81 MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 333(7572), 779 (2006).
- 82 MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology 243(1), 132–139 (2007).
- 83 Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br. J. Surg. 95(2), 229–236 (2008).
- 84 . Evaluating mesorectal lymph nodes in rectal cancer before and after neoadjuvant chemoradiation using thin-section T2-weighted magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 71(2), 456–461 (2008).
- 85 . MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy – implications for induction chemotherapy? Int. J. Radiat. Oncol. Biol. Phys. 87(3), 505–511 (2013).
- 86 Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur. J. Surg. Oncol. 36(11), 1061–1065 (2010).
- 87 Complete pathologic response in the primary of rectal or colon cancer treated with FOLFOX without radiation. J. Clin. Oncol. 28(Suppl.), Abstract 3649 (2010).
- 88 Neoadjuvant capecitabine, oxliplatin, and bevacizumab (CAPOX-B) in intermediate-risk rectal cancer (RC) patients defined by magnetic resonance (MR): GEMCAD 0801 trial. J. Clin. Oncol. 30(Suppl.), Abstract 3586 (2012).
- 89 Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J. Clin. Oncol. 43(10), 964–971 (2013).•• Phase II study of neoadjuvant chemotherapy without radiotherapy in locally advanced rectal cancer.
- 90 Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J. Clin. Oncol. 32(6), 32–518 (2014).•• Phase II study of neoadjuvant chemotherapy without radiotherapy in locally advanced rectal cancer.
- 91 Neoadjuvant FOLFOX6 Chemotherapy With or Without Radiation in Rectal Cancer (FOWARC). http://clinicaltrials.gov/ct2/show/NCT01211210.
- 92 Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery. http://clinicaltrials.gov/ct2/show/NCT01515787.
- 93 Bevacizumab And Combination Chemotherapy in Rectal Cancer Until Surgery (BACCHUS). http://clinicaltrials.gov/ct2/show/NCT01650428.
- 94 . The contemporary (over)treatment of rectal cancer: the goldilocks effect. Dis. Colon Rectum 57(3), 403–406 (2014).
































