Diagnostic use of epitope detection in monocytes blood test for early detection of colon cancer metastasis
Abstract
A follow-up strategy in cancer aftercare can result in early detection of metastasis and/or recurrence. Therefore, sensitive and reliable diagnostic tests that are easy to perform are needed. Here, the authors present the combined use of the epitope detection in monocytes (EDIM)–TKTL1 and EDIM–Apo10 blood test in aftercare monitoring of a patient with colon carcinoma. Whereas the established tumor markers CEA and CA19-9 did not indicate metastasis even at a timepoint where clinical signs and imaging techniques already demonstrated metastasis, the combined application of the EDIM–TKTL1 and the EDIM–Apo10 blood tests was positive 9 months before detection of metastasis. These findings – taken together with recently published evaluation data of the EDIM–TKTL1 blood test – suggest that the combined application of the EDIM–TKTL1 and the EDIM–Apo10 blood tests might indicate metastasis earlier than established tumor markers and could serve as sensitive and noninvasive methods that might be used for early detection of colon cancer metastasis.
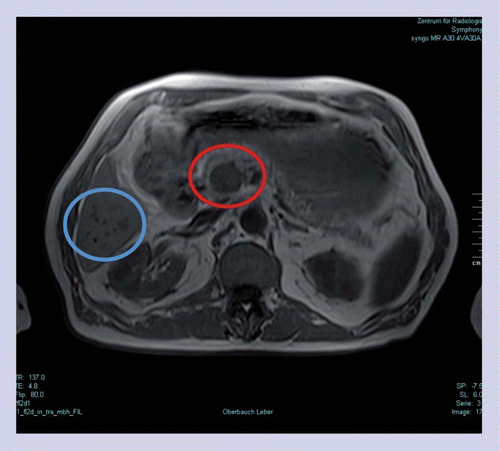
Two metastases in the area of the pancreatic head infiltrating pancreatic tissue were confirmed: a rounded, 2.5-cm large metastasis between mesenteric vessels and the pancreas (red); and a 1.5-cm large metastasis proceeding along the vessels over 6 cm in length, which is not visible in this sectional plane. Furthermore, six small liver metastases (<12 mm) were determined in segment VI (blue).
Sensitive and reliable diagnostics are fundamental for early detection of cancer, as well as for monitoring therapy success. The early detection of cancer and its precursors improves the chances of healing. In addition, the early detection of recurrence and/or metastasis can improve survival and prognosis or can even – in cases of complete resection – result in full recovery. Tumor markers have been established for some types of cancer, such as colorectal, breast and prostate cancer; however, new tumor markers with high sensitivity and specificity are still needed, especially for those tumor entities where no tumor markers are available. Moreover, diagnostic tests should be applicable for more than one tumor entity – ideally for all types of tumors – in an easy and noninvasive way.
A blood test, which is based on published technology of epitope detection in monocytes (EDIM) [1], takes advantage of the fact that activated macrophages that have phagocytozed tumor cells can be detected using markers specific for activated monocytes (CD14 and CD16). Simultaneously, intracellular-presented tumor cell-derived proteins can be detected. Altogether this allows detection and characterization of phagocytozed tumor cells [2–4]. From the whole subset of proteins expressed in tumor cells, two specific proteins have been selected owing to their role in tumorigenesis.
The transketolase-like-1 (TKTL1) protein represents a marker that indicates upregulation of the nonoxidative arm of the pentose phosphate pathway and increased glucose metabolism. The oxidative as well as the nonoxidative arm of the pentose phosphate pathway contribute to tumor metabolism, stimulate malignant transformation, are associated with tumor proliferation and progression, enhanced angiogenesis and favor tumor migration and metastasis [5]. TKTL1 expression is associated with poor prognosis in cancer patients [6,7]. In rectal cancer patients with high TKTL1 levels, the development of metastases or local recurrence was significantly increased compared with patients with low TKTL1 levels [7]. Apo10 overexpression has been detected in different types of tumors and is concomitant with inhibition of apoptosis and, thus, is indicative of abnormal cell proliferation [2,3].
Here, the authors present the first proof of concept of the combined application of EDIM–TKTL1 and EDIM–Apo10 blood tests in aftercare monitoring of a patient with colon carcinoma.
Case report
A 71-year-old man was suffering from diabetes mellitus Type II, hypertension, hypercholesterolemia and sigma diverticulosis. In September 2007 a fecal occult blood test revealed a positive result. Using a colonoscopy, a 3 × 3 cm tumor mass was identified. A right hemi colectomy was performed 2 weeks later and histological examination of the excised tumor tissue revealed a colon carcinoma pT3, N0, M0, R0 and G2. According to the German guidelines on colorectal cancer, no chemotherapy or radiation therapy was performed [8].
Physical examinations and abdomen ultrasound examinations were performed every 6 months according to the guidelines and levels of CEA and CA 19-9 tumor markers were regularly monitored. In addition, the EDIM–TKTL1 and the EDIM–Apo10 blood tests were performed every 3 months starting in February 2011.
All results from physical and abdomen ultrasound examinations were without findings. In addition, levels of CEA and CA19-9 were within the normal range at all measuring times from November 2008 to November/December 2011 (Table 1).
In contrast to these results, at least one of both scores of the EDIM–TKTL1 and the EDIM–Apo10 blood tests was elevated at all measuring times from February 2011 until March 2012 (Table 1). In September 2011, a colonoscopy, which was performed according to the guidelines, did not show any recurrence.
In November 2011, 2 months after the colonoscopy, the patient was hospitalized owing to persistent serious bloody diarrhea and collapse conditions. Anemia with a hemoglobin of 4 g/dl was diagnosed and, therefore, metastases were suspected. MRI of the abdomen showed a 41 × 27 mm hypovascularized metastasis located close to the pancreas and liver metastasis was suspected. Again, at this timepoint, CEA and CA19-9 levels still showed negative results, whereas the EDIM–TKTL1 and the EDIM–Apo10 blood tests both showed positive results (Table 1).
To clarify the patient’s operability, a native MRI of the liver and pancreas was carried out in December 2011 confirming multiple metastases in the liver and pancreas (Figure 1), which were considered inoperable. The patient was conducted to palliative chemotherapy in January 2012. In March 2012, 4 months after the first detection of multiple metastases in the liver and pancreas, the CEA level was within the threshold range and the CA19-9 level was elevated but still negative (Table 1).
Discussion
More than 1 million people worldwide are diagnosed with colorectal cancer every year. As of 2008 it is the second most common cause of cancer in women and the third most common in men [9]. Colorectal cancer – similar to some of the most common cancer types such as breast, cervical or oral cancer – has a higher cure rate when detected early and treated according to best practices [10]. In addition, an intensive follow-up strategy leads to mortality reduction [11]. Therefore, a noninvasive blood test that allows for reliable, early detection of malignancies and progression thereof would be the easiest way to detect cancer and monitor disease progression.
In colorectal cancer treatment guidelines, the CEA test is recommended in aftercare, not as a solitary marker, but as part of a periodical follow-up strategy also including modern imaging techniques, endoscopy and physical examination [8,11]. However, recurrence or metastasis is not indicated by elevated CEA levels in all patients [12]. In addition, CA19-9, which can be elevated in many types of gastrointestinal cancer, such as colorectal cancer, esophageal cancer and hepatocellular cancer, is often used in combination with CEA to manage patients with colorectal cancer. However, currently there is insufficient evidence to support its use [13]. In the patient presented here, CEA and CA19-9 were not able to detect metastasis.
The EDIM technology has been chosen for the detection of the biomarkers TKTL1 and Apo10 in order to establish diagnostic tests with high sensitivity and high specificity. The TKTL1 protein represents a key enzyme in the nonoxidative arm of the pentose phosphate pathway [5]. Although the exact function of TKTL1 is not yet characterized, several in vitro and in vivo results clearly demonstrate that TKTL1 protein strongly influences glucose metabolism and reduces sensitivity toward radical and apopotosis induction in cancer cells. Wanka et al. demonstrated that TIGAR protects glioma cells from starvation-induced cell death only when TKTL1 protein is present. Inhibition of TKTL1 protein led to cell death [14]. Furthermore, TKTL1 has been found to be upregulated in several tumor types, and it has already been shown that upregulation of TKTL1 in patients with colorectal and urothelial cancer is associated with poor prognosis [6,7].
The diagnostic efficacy of the EDIM–TKTL1 test has been evaluated in a comparison study with fluorodeoxyglucose-PET/computed tomography (CT) examinations in 240 patients with 17 different tumor entities. The study revealed a good concordance of 90% between the EDIM–TKTL1 blood test and fludeoxyglucose-PET results and the sensitivity and specificity of the EDIM–TKTL1 blood test was found to be 94 and 81%, respectively. According to this evaluation, EDIM–TKTL1 result scores <119 are defined as TKTL1 ‘negative’ and those ≥119 as TKTL1 ‘positive’ [4].
Apo10 has been selected as a biomarker, which, according to the authors own yet unpublished data of 25 different tumor entities, appear to indicate inhibition of apoptosis and abnormal cell proliferation [Coy JF, Unpublished Data]. Apo10 overexpression represents a very early event during malignant transformation from normal cells to tumor cells and, therefore, might possibly be a useful biomarker for the early detection of neoplasias. Similar to the EDIM–TKTL1 evaluation, the EDIM–Apo10 blood test has been evaluated using 10,000 blood samples from patients with confirmed or suspected malignancies, as well as from blood donors to determine the distribution of Apo10 scores in the normal population. Furthermore, Apo10 scores have been evaluated in cancer patients with prostate, bladder, lung, gastric, colon, breast, kidney, oral squamous cell and pancreas carcinomas, as well as melanomas, sarcomas, glioblastomas and leukemias, confirming the overexpression of Apo10 in patients with solid tumors and leukemia. These data revealed that EDIM–Apo10 scores <100 are regarded as normal. A pilot study in prostate and breast cancer patients has shown a good concordance of Apo10 overexpression and the confirmed presence of a tumor [2,3]. Therefore, combined use of biomarkers Apo10 and TKTL1 in the EDIM technology should offer the possibility of detecting abnormal cell proliferation (Apo10) and upregulated glucose metabolism (TKTL1), indicating neoplasias and the degree of malignancy [2,4].
The case presented here can be taken as a first proof of concept of the combined application of the EDIM–TKTL1 and EDIM–Apo10 blood tests in colon cancer aftercare. The authors results further support the tests’ potential role in cancer diagnostics. While in this patient, the CEA and CA19-9 levels were below the threshold at all measuring times from November 2008 until March 2012 and, therefore, did not indicate metastasis, both the EDIM–TKTL1 and the EDIM–Apo10 values were positive 9 months before the detection of metastasis based on imaging results. Whereas EDIM–Apo10 was positive at all measuring times, the EDIM–TKTL1 was negative in April 2011, indicating that a combination of EDIM–Apo10 and EDIM–TKTL1 could possibly lead to a better diagnostic performance as detection of upregulated glucose metabolism alone might not be sufficient for the early detection of recurrences or metastasis.
It might be considered a limitation of this case report that MRI scans were not applied at the same time when EDIM–TKTL1 and EDIM–Apo10 values were elevated for the first time. However, it has to be kept in mind that the classical tumor markers were negative at the first measuring time (February 2011) and the evaluation data on the EDIM–TKTL1 blood test were not yet published. Therefore, elevated EDIM–TKTL1 and EDIM–Apo10 scores were unfortunately not a sufficient indication to initiate MRI or contrast-enhanced CT scans in daily clinical practice. Following the second elevated EDIM–TKTL1 and EDIM–Apo10 results in August 2011, a colonoscopy was performed in September 2011 to detect recurrence in the colon as recommended by the German guidelines. Since the colonoscopy yielded a negative result, further imaging techniques were not applied. Therefore, metastasis in the pancreatic region could not be detected earlier and EDIM–TKTL1 and EDIM–Apo10 results could only be finally verified in November/December 2011 by MRI. It should be noted that even at this timepoint the classical tumor markers were still negative, while both the EDIM–TKTL1 and the EDIM–Apo10 blood test were positive.
Imaging technologies such as MRI or fludeoxyglucose-PET/CT examinations are important to gain detailed information about the localization and distribution of malignancies in the human body. As shown in this case report, the EDIM–TKTL1 and EDIM–Apo10 blood tests might help to better determine the right time for further imaging examinations at an early stage. Therefore, the EDIM–TKTL1 and EDIM–Apo10 blood tests – in combination with imaging technologies – might help to optimize cancer diagnosis and cancer aftercare treatment. As shown in this report, this might be important, especially in patients who do not show elevated levels of established tumor markers like CEA or CA19-9 during aftercare or at initial diagnosis.
In summary, the case report presented here could serve as a proof of concept for the combined use of EDIM–TKTL and EDIM–Apo10 blood tests in aftercare for early detection of recurrences and metastasis. Therefore, the observed data suggest that the combination of EDIM–TKTL1 and EDIM–Apo10 blood tests might be used for the detection of metastasis in colorectal cancer patients in an aftercare situation. Of course, further prospective clinical studies with large patient cohorts are needed to characterize the performance of EDIM–TKTL1 and EDIM–Apo10 in aftercare.
Conclusion & future perspective
This case report represents a first proof of concept that the EDIM–TKTL1 and EDIM–Apo10 blood tests might serve as sensitive and noninvasive methods and might be used for early detection of colon cancer metastasis. This needs to be explored in further evaluation studies.
| Date | TKTL1 | Apo10 | CA19-9 | CEA | Colonoscopy | Surgery/pathology | MRI abdomen | MRI liver/pancreas |
|---|---|---|---|---|---|---|---|---|
| September 2007 | + | |||||||
| October 2007 | + (colon carcinoma) | |||||||
| November 2008 | - (<5.0) | - (0.8) | ||||||
| November 2009 | - (0.6) | |||||||
| February 2011 | + (136) | + (173) | - (10.4) | - (1.3) | ||||
| April 2011 | - (115) | + (210) | ||||||
| August/September 2011 | + (122) | + (111) | - (6.0) | - (1.4) | - | |||
| November/December 2011 | + (166) | + (113) | - (12.0) | - (1.5) | + | + | ||
| March 2012 | + (150) | + (131) | - (35.4) | +/- (3.0) |
Acknowledgements
The authors would like to thank H Hofmann and O Feyen, both from TAVARLIN AG, Darmstadt, Germany for support in drafting and revising this manuscript. Thanks to R Tomzcak, Institute for Radiology and Nuclear Medicine, B Friedrichshall, Germany for providing the MRI image and J Rieger, Senckenberg Institute of Neurooncology and Clinic for Neurosurgery, Johann-Wolfgang Goethe University, Frankfurt, Germany, for critical reading of the manuscript.
Financial & competing interests disclosure
JF Coy is an employee and shareholder of TAVARLIN AG, Darmstadt, Germany. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was utilized in the production of this manuscript. E Stetzer, QuintesScience, Darmstadt, Germany, provided editorial support, which was funded by TAVARLIN AG.
References
- 1 Japink D, Leers MP, Sosef MN, Nap M. CEA in activated macrophages. New diagnostic possibilities for tumor markers in early colorectal cancer. Anticancer Res.29,3245–3251 (2009).
- 2 Arnholdt J. Therapy monitoring and early detection of metastasis using tumor protein detection in macrophages. Anticancer Res.31,1957–2016 (2011).
- 3 Rotmann AR, Hofmann HA, Coy JF. Apo10 – a new biomarker for early detection of disorders of cell proliferation and solid tumours. Presented at: XX FIGO World Congress of Gynecology & Obstetrics. Rome, Italy, 7–12 October 2012.
- 4 Feyen O, Coy JF, Prasad V et al. EDIM–TKTL1 blood test: a noninvasive method to detect upregulated glucose metabolism in patients with malignancies. Future Oncol.8,1349–1359 (2012).
- 5 Riganti C, Gazzano E, Polimeni M et al. The pentose phosphate pathway: an anti-oxidant defense and a cross-road in tumor cell fate. Free Radic. Biol. Med.53,421–436 (2012).
- 6 Langbein S, Zerilli M, Zur Hausen A et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br. J. Cancer94,578–585 (2006).
- 7 Schwaab J, Horisberger K, Strobel P et al. Expression of transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. Biomed. Cancer11,363 (2011).
- 8 Schmiegel W, Pox C, Reinacher-Schick A et al. S3 guideline ‘colorectal carcinoma’. Results from evidence based consensus conferences (Feb 2004 and June 2007). Z. Gastroenterol.46,1–73 (2008).
- 9 Jemal A, Bray F, Center MM et al. Global cancer statistics. CA Cancer J. Clin.61,69–90 (2010).
- 10 WHO . Cancer. Fact Sheet No. 297. WHO, Geneva, Switzerland (2012).
- 11 Rodríguez-Moranta F, Saló J, Arcusa A et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J. Clin. Oncol.24,386–393 (2006)
- 12 Hara M, Kanemitsu Y, Hirai T et al. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients with Dukes C colorectal cancer: analysis with likelihood ratio and posttest probability in a follow-up study. Dis. Colon. Rectum51,1675–1680 (2008).
- 13 Locker G, Hamilton S, Harris J et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol.24,5313–5327 (2006).
- 14 Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by upregulating respiration and improving cellular redox homeostasis. J. Biol. Chem.287,33436–33446 (2012).
































