Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment
Abstract
ESR1 mutation (ESR1m) is a frequent cause of acquired resistance to aromatase inhibitor (AI) plus cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i), which is a first-line therapy for hormone-receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC). Camizestrant is a next-generation oral selective estrogen receptor degrader (SERD) that in a phase II study significantly improved progression-free survival (PFS) over fulvestrant (also a SERD) in ER+/HER2- ABC. SERENA-6 (NCT04964934) is a randomized, double-blind, phase III study evaluating the efficacy and safety of switching from an AI to camizestrant, while maintaining the same CDK4/6i, upon detection of ESR1m in circulating tumor DNA before clinical disease progression on first-line therapy for HR+/HER2- ABC. The aim is to treat ESR1m clones and extend the duration of control of ER-driven tumor growth, delaying the need for chemotherapy. The primary end point is PFS; secondary end points include chemotherapy-free survival, time to second progression event (PFS2), overall survival, patient-reported outcomes and safety.
Plain language summary
Why will we perform this study? Patients with advanced breast cancer in which the cancer cells have the receptor for the hormone estrogen and/or progesterone are typically treated with an aromatase inhibitor, a hormone therapy that decreases estrogen being made in the body, together with an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), a drug that blocks the growth of cancer cells. Although cancers usually respond to treatment initially, the cancer cells eventually change, so the drug combination no longer works. For example, mutation of the estrogen receptor (referred to as ESR1m) can stop aromatase inhibitors from working. Camizestrant is an investigational drug that blocks estrogen receptors, including mutated receptors, reducing the growth and spread of cancer. Here we describe the SERENA-6 clinical trial, which is testing camizestrant as a treatment for patients with breast cancer with ESR1m. How will we perform this research? The phase III SERENA-6 trial will use blood tests to monitor if patients with breast cancer develop ESR1m while being treated with an aromatase inhibitor and a CDK4/6 inhibitor. If ESR1m is detected, yet the disease is stable, participants will be randomly assigned to either continue with the same aromatase inhibitor or switch to camizestrant while continuing with the same CDK4/6 inhibitor. The study will assess whether switching to camizestrant prolongs the time before the cancer grows, spreads or worsens. It will also assess the length of time that participants live for versus those who continue with an aromatase inhibitor.
Clinical Trial Registration:NCT04964934 (ClinicalTrials.gov)
Tweetable abstract
SERENA-6 (NCT04964934) is an ongoing randomized, double-blind, phase III trial of camizestrant + CDK4/6i in ESR1-mutant HR+/HER2-negative advanced breast cancer during first-line treatment.
Hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) tumors account for more than two-thirds of all breast cancers [1]. Endocrine therapies that suppress estrogen receptor (ER) alpha signaling are the treatment backbone for HR+/HER2- advanced breast cancer (ABC) [2]. Aromatase inhibitors (AIs; letrozole, anastrozole and exemestane) inhibit estrogen synthesis in postmenopausal women and reduce ER signaling via lowering estrogen levels [3]. Selective estrogen receptor degraders (SERDs) directly bind to ER, block ER signaling, and target ER for degradation. Most SERDs also act as complete antagonists, while selective ER modulators (SERMs, e.g., tamoxifen) may antagonize or have partial agonism for ER, depending on the tissue context [3].
First-line standard-of-care (SOC) for HR+/HER2- ABC (comprising locally advanced non-resectable or metastatic breast cancer [mBC]) typically includes endocrine therapy combined with an inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6i) [2,4,5]. Compared with endocrine monotherapy, the combination of an endocrine agent with a CDK4/6i extends time on first-line therapy and overall survival (OS) [6]. For example, a meta-analysis of more than 2000 patients with HR+/HER2- ABC showed the median progression-free survival (PFS) with an AI plus CDK4/6i to be 28.0 months versus 14.9 months for an AI with placebo [7]. The vast majority of patients with HR+/HER2- ABC receive first-line treatment with CDK4/6i plus AI, and this regimen is recommended by most clinical guidelines [4,8–11]. Three different CDK4/6i are in widespread use (abemaciclib, palbociclib and ribociclib); due to differences in toxicity profiles, patients who do not tolerate one CDK4/6i may experience less toxicity with another [2,6].
Despite the proven efficacy of endocrine therapy plus CDK4/6i for ABC, resistance invariably develops [6]. The optimal subsequent treatments following progression on first-line endocrine therapy plus CDK4/6i remains to be established. However, once HR+/HER2- ABC has progressed, response to subsequent lines of endocrine-based therapy can be diminished. For example, in the EMERALD study, a median PFS of 1.9 months was observed in patients receiving SOC endocrine therapy (an AI or the currently licensed SERD, fulvestrant) (n = 238) with prior CDK4/6i exposure and progressed on one or two previous lines of endocrine therapy (59 and 41% of participants, respectively) [12]. The VERONICA study showed a similar median PFS of 1.9 months with fulvestrant therapy in patients (n = 51) who experienced disease recurrence or progression during or after CDK4/6i therapy for at least 8 weeks [13]. Consequently, there is a significant unmet need to identify therapeutic approaches that extend the duration of first-line endocrine-based therapy, to enable patients with HR+/HER2- ABC to derive clinical benefit from the treatment as well as maintain their health-related quality of life. Delaying initiating chemotherapy would also be favorable.
ESR1 mutation in acquired resistance to AI therapy
Endocrine therapy resistance is frequently associated with the emergence of alterations in the genes involved in ER signaling, growth factor receptor signaling (such as the PI3K/AKT/PTEN signaling pathway), cell cycle regulation, and apoptosis. ESR1 encodes ER alpha, and resistance to AI therapy is frequently associated with activating mutations in the region of ESR1 that encodes the ER ligand-binding domain (referred to as ESR1 mutations [ESR1m]), which cause constitutive (estrogen-independent) activation of ER (Figure 1) [14,15].

(A) wt-ER and m-ER signaling. (B) AIs suppress wt-ER signaling via the downregulation of estradiol synthesis, but do not affect estrogen-independent signaling by m-ER. (C) Camizestrant (ngSERD) binds to wt-ER and m-ER and targets both for proteosome-mediated degradation. ngSERD binding also prevents estradiol binding and the formation of the functional wt-ER or m-ER-containing transcription factor complex, thereby preventing ER-driven transcriptional changes.
AI: Aromatase inhibitor; CoA: Coregulator; ER: Estrogen receptor; ERE: Estrogen response element; ESR1m: Estrogen receptor 1 gene with an activating mutation in the region that encodes the ligand-binding domain; m-ER: The mutated form of the estrogen receptor alpha protein, the product of ESR1m; ng: Next generation; SERD: Selective estrogen receptor degrader.
ESR1m are rare in endocrine-therapy-naive ER+/HER2- ABC, and the frequency is even lower if an AI has not been administered in the adjuvant setting (estimates are 3–6% [16,17]). In contrast, studies that enrolled patients after first-line AI therapy found that approximately 30% of patients have ESR1m-positive ABC (Table 1) [12,18–23]. The frequency at which ESR1m is detected is also influenced by the length of time for which patients received an AI, as shown by the PADA-1 study. This study serially screened circulating tumor DNA (ctDNA) from 1017 patients receiving an AI with palbociclib as first-line treatment for mBC and showed that 279 patients developed detectable ESR1m during the first step of the study (ESR1m detection phase). These 279 patients accounted for 27% of the whole patient population, including patients still on treatment and with no tumor progression at the time of the analysis; 172 (17%) patients were randomly assigned to treatment [24]. In the PADA-1 study, ESR1m was less common during the first 6 months of standard first-line therapy than after 6 months of treatment (p < 0.001) suggesting that ESR1m is rarely involved in primary resistance but may represent the most prevalent mechanism of acquired resistance [25]. PADA-1 also showed a median time of 14.2 months (range 2.8–47.1 months) from starting first-line AI plus CDK4/6i therapy (n = 1017) to the detection of ESR1m in ctDNA [26]. Serial screening of ctDNA from plasma has suggested that ESR1m is detectable approximately 3–6 months before HR+ ABC progresses according to clinical and/or radiological criteria [27,28].
| Trial | Tumor characteristics | Timing of test | Test (sample) | ESR1m frequency | N, ESR1m/total | Ref. |
|---|---|---|---|---|---|---|
| MONARCH 3 | Endocrine-therapy-naive HR+/HER2- ABC treated with 1L AI monotherapy (control arm) | End of 1L AI treatment | NGS (plasma) | 31% | NR | [20] |
| EMERALD | One or two previous lines of endocrine therapy, at least one in combination with CDK4/6i | Start of 2L or 3L treatment | NGS (plasma) | 48% | 228/477 | [12] |
| GuardantINFORM database | At least one previous AI therapy | Post-AI therapy | NGS (plasma) | 31% | 2044/6541 | [21] |
| SoFEA/EFECT | HR+ mBC that had progressed on previous AI monotherapy | Start of 2L treatment | ddPCR (plasma) | 30% | 151/383 | [18] |
| BOLERO-2 | HR+ ABC that had progressed on previous AI monotherapy | Start of 2L or 3L treatment | ddPCR (plasma) | 29% | 156/541 | [19] |
| PEARL | AI-resistant HR+/HER2- mBC | Start of 2L or 3L treatment | ddPCR (plasma) | 29% | 164/557 | [22] |
| PALOMA-3† | HR+/HER2- mBC that had relapsed or progressed on previous AI or tamoxifen monotherapy | Start of 2L or 3L treatment | ddPCR (plasma) | 26% | 114/445 | [23] |
In women with ABC, detection of ESR1m ctDNA is associated with substantially shorter PFS on subsequent AI-based therapy compared with women without detectable ESR1m [17]. Analysis of patients in the phase III SoFEA and EFECT trials of exemestane versus fulvestrant (for patients with HR+ ABC that had progressed on first-line AI) showed a median PFS of 2.4 months (n = 42) versus 4.8 months (n = 121) with exemestane for disease that was ESR1m-positive versus ESR1m-negative, respectively [18]. Furthermore, the 1-year OS rate was 62% for participants with ESR1m-positive disease, compared with 79% for those with ESR1m-negative disease. In the control arm of BOLERO-2, which recruited patients with HR+ ABC that had progressed on AI previously, exemestane therapy showed a median PFS of 2.8 months for ESR1m-positive disease (n = 61) versus 3.9 months for ESR1m-negative disease (n = 128) [19]. Additionally, in the overall population of BOLERO-2, the median OS was 32.1 months for participants without detectable ESR1m (n = 385) compared with 20.7 months for those with ESR1m-positive disease (n = 156) [19]. In the phase II BYLieve trial in PIK3CA mutation-positive HR+/HER2- ABC, a cohort of participants who received letrozole plus the PI3K inhibitor alpelisib showed a median PFS of 4.6 months versus 7.0 months for ESR1m-positive (n = 25) versus ESR1m-negative disease (n = 72) (hazard ratio [HR] 0.55, 95% CI 0.32–0.92, nominal p = 0.02) [29].
In addition to causing AI resistance, preclinical analysis of gene transcription profiles shows that ESR1m upregulates genes that promote metastasis [30], with ESR1m acquisition possibly associated with more aggressive features such as the development of visceral metastasis [31,32]. There is evidence that tumors that acquire ESR1m also gain additional genetic complexity that further suppresses the response to additional lines of therapy [33]. Indeed, genetic complexity was associated with lower benefit from fulvestrant in the plasmaMATCH study [34]. In exploratory analyses of ctDNA from the phase III PALOMA-3 trial, which investigated palbociclib plus fulvestrant versus fulvestrant in patients with HR+/HER2- ABC that had progressed on prior endocrine therapy, detection of ESR1m was prognostic for shorter OS regardless of treatment (HR: 1.58, 95% CI: 1.22–2.06) [35].
Considering the available evidence, ESR1m is a promising clinical resistance biomarker in HR+ mBC [33]. The European Society for Medical Oncology (ESMO) Precision Medicine Working Group noted that ESR1m might be more actionable when detected sooner by ctDNA rather than later at radiological progression [36].
SERDs, novel SERDs and ESR1m
SERDs have a distinct mechanism of action compared with AIs because they directly bind to the ligand-binding domain of ER alpha to antagonize estrogen binding and induce proteosome-mediated degradation of ER alpha (Figure 1). Fulvestrant was the first licensed SERD and is delivered by intramuscular injection [3,37]. Clinical data suggest that fulvestrant remains active, albeit modestly effective, for HR+/HER2- ABC carrying certain (but not all) ESR1m variants. A combined analysis of the SoFEA and EFECT trials showed a median PFS of 3.9 versus 4.1 months and a 1-year OS rate of 80% versus 81% for fulvestrant treatment of HR+ ABC with detectable ESR1m (n = 73) and without detectable ESR1m (n = 147), respectively [18]. In SOLAR-1, the median PFS of patients receiving fulvestrant plus the PI3K inhibitor alpelisib with versus without detectable ESR1m was 12.0 months (n = 13) versus 11.0 months (n = 107), respectively [38]. Similarly, in PALOMA 3, the median PFS reported in patients receiving fulvestrant plus palbociclib with versus without detectable ESR1m was 11.3 months (n = 69) versus 11.1 months (n = 154), respectively [35]. However, these data were generated in patients who were not previously treated with CDK4/6i. Recent data suggest that prior CDK4/6i exposure is a prognostic factor for fulvestrant efficacy in patients with ESR1m-positive disease. Post-CDK4/6i treatment, the median PFS with fulvestrant was in the range of 2–3 months [12]. These results support early initiation of a SERD in patients with ESR1m-positive disease before disease progression post-CDK4/6i treatment [12].
The PADA-1 trial serially tested ctDNA for ESR1m and then examined the clinical benefit of switching patients from an AI plus palbociclib to fulvestrant plus palbociclib when ESR1m was detected in ctDNA but before clinical disease progression while receiving CDK4/6i plus AI [24,39]. Data from PADA-1 showed longer PFS (from the point of switching) for patients with ESR1m-positive tumors who switched to fulvestrant than for those who continued with an AI (median 11.9 versus 5.7 months; HR: 0.61, 95% CI: 0.43–0.86; p = 0.004; n = 172) [24].
Fulvestrant was approved as a breast cancer therapy in 2002 and was the first-in-class SERD. Although fulvestrant has proven efficacy and well-characterized safety, it has suboptimal pharmacokinetic properties, including low aqueous solubility that requires regular, potentially painful, monthly intramuscular delivery [3,37]. In addition, there is evidence from preclinical models that the efficacy of fulvestrant is reduced by certain types of ESR1m, especially Y537S [40]. These limitations stimulated the development of SERDs with improved bioavailability, target engagement, and ESR1m coverage. The first oral agents did not show better efficacy than fulvestrant in preclinical models and/or early-phase clinical trials [41]. Continued research led to the development of several SERDs, which have shown superior efficacy to fulvestrant in preclinical models, including patient-derived xenografts with the Y537S ESR1m [40]. In the clinical trial setting, several oral SERDs are in late-stage development including camizestrant (AZD9833), giredestrant (GDC-9545), and imlunestrant (LY3484356), and the SERM/SERD elacestrant (RAD1901) [3,37,41,42]. Amcenestrant (SAR439859), another oral SERD, was until recently in late-stage development, but global clinical development was stopped following negative clinical trial results [43].
Elacestrant is the first oral SERM/SERD with results from a phase III trial (EMERALD); elacestrant (n = 239) versus SOC endocrine therapy (n = 238) in patients with HR+/HER2- ABC whose disease had progressed on prior endocrine therapy plus CDK4/6i [12]. The trial showed that elacestrant significantly improved PFS (blinded independent central review; HR: 0.70; 95% CI: 0.55–0.88; p = 0.002; elacestrant versus SOC: median PFS 2.8 months versus 1.9 months, respectively) [12]. Notably, the PFS benefit of elacestrant was greatest in patients with detectable ESR1m ctDNA (48% of the overall population; HR: 0.55, 95% CI: 0.39–0.77; p = 0.0005; elacestrant versus SOC: median PFS 3.8 months versus 1.9 months, respectively) [12]. Only 149 OS events (of which only 68 events were in patients with ESR1m) had occurred at the primary analysis of EMERALD, but the immature data suggested that elacestrant may provide OS benefit over SOC in the ESR1m-positive subgroup (HR: 0.59, 95% CI: 0.36–0.96; p = 0.03 [non-significant]) [12]. Elacestrant reported a manageable safety profile with mostly Grade 1 or 2 adverse events (AEs) [12].
Single-arm, early-phase clinical trials of novel oral SERDs have shown encouraging antitumor activity in both ESR1m and wild-type ESR1 populations, including in patients previously treated with or without CDK4/6i [3,37,44]. In the phase II setting, these compounds as a monotherapy, have been compared with available standard endocrine therapy in patients who have progressed on prior endocrine therapy in the ABC setting. The phase II acelERA trial did not meet its primary end point of improved PFS for giredestrant versus endocrine treatment of physician's choice (TPC) for patients with ER+/HER2- ABC who had received one or two lines of previous endocrine therapy [45]. The PFS HR with giredestrant versus TPC was numerically improved in patients with detectable ESR1m ctDNA at baseline (39% of the overall population; HR: 0.60, 95% CI: 0.35–1.03) [45]. Giredestrant was reported to be well tolerated with a similar safety profile compared with TPC [45]. These findings were numerically aligned with the results of EMERALD, where PFS benefit of elacestrant was greatest in patients with detectable ESR1m ctDNA [12]. This suggests the presence of ESR1m is a marker indicating tumor growth still driven by ER signaling in patients with acquired resistance to current endocrine therapies.
In early-phase clinical studies, the next-generation oral SERD camizestrant (as monotherapy or in combination with palbociclib or abemaciclib) has shown promise in offering improvements to SOC endocrine therapy, including in patients with detectable ESR1m (see next section for details) [46–48]. Most recently, in the randomized phase II SERENA-2 trial camizestrant significantly improved PFS versus fulvestrant in post-menopausal patients with ER+ ABC, previously treated with endocrine therapy [49]. The results from two ongoing phase III studies, SERENA-4 (NCT04711252) and SERENA-6 (NCT04964934) will further elucidate the role of camizestrant in treating patients with HR+/HER2- ABC.
SERENA-6
A phase III, double-blind, randomized study to assess switching to camizestrant plus CDK4/6i versus continuing an AI plus CDK4/6i in patients with HR+/HER2- ABC with detectable ESR1m but without clinical disease progression during first-line treatment with an AI plus CDK4/6i: a ctDNA-guided early switch study sponsored by AstraZeneca.
Background & rationale
Camizestrant (AZD9833) is a potent next-generation oral SERD and pure ER antagonist [50] (Figure 1) that was designed to improve on first-generation oral SERDs [3,37]. The pharmacokinetic profile of camizestrant is consistent with once-daily oral dosing in patients [46,48,51,52]. Camizestrant has demonstrated antitumor activity in a wide range of ER+ breast cancer cell lines and patient-derived xenograft models, including those with wild-type ESR1 and ESR1m Y537S and D538G [50,53]. In the phase I SERENA-1 trial, camizestrant was associated with encouraging clinical activity as monotherapy or in combination with CDK4/6i in patients with ER+/HER2- ABC who had received one or more previous lines of endocrine therapy, including fulvestrant and/or a previous CDK4/6i [46–48,52]. Furthermore, ESR1m detected in ctDNA at baseline was reduced to undetectable levels in most patients receiving camizestrant monotherapy or in combination with a CDK4/6i, including participants with ESR1m Y537S [46–48].
In the randomized phase II SERENA-2 trial, treatment with camizestrant (at 75 and 150 mg doses) demonstrated a statistically significant improvement in PFS benefit versus fulvestrant in post-menopausal patients with ER+ ABC, previously treated with endocrine therapy [49]. The benefit in PFS extended to subgroups with detectable ESR1m at baseline where camizestrant 75 mg (the recommended phase III dose of camizestrant selected for SERENA-6) reduced the risk of disease progression over fulvestrant with a HR of 0.33 (95% CI: 0.18–0.58). Consistent with other recent published data, fulvestrant in patients with detectable ESR1m at baseline had a median PFS of 2.2 months.
Monotherapy camizestrant 75 mg has shown a tolerable safety profile in SERENA-1 with the majority of possibly camizestrant-related (investigator assessed) AEs reported as Grade 1 [48]. Furthermore, no patient required camizestrant discontinuation due to possibly camizestrant-related AEs [48]. Camizestrant 75 mg was similarly well tolerated in SERENA-2 with no new safety signals identified [49]. The safety profile of camizestrant plus palbociclib or abemaciclib was consistent with the known profile of each agent given as monotherapy, with no patient requiring camizestrant dose reduction [46,47]. No clinically significant drug–drug interactions have been observed when camizestrant 75 mg is dosed with palbociclib or abemaciclib [46,47].Studies investigating the combination of camizestrant plus ribociclib are ongoing.
Across SERENA-1 and SERENA-2, low grade AEs of visual effects and heart rate reduction were reported, and are known to be associated with camizestrant [46–49,52]. In SERENA-1, all possibly camizestrant-related (investigator-assessed) AEs of visual effects and heart rate reduction with camizestrant 75 mg as either monotherapy or in combination with palbociclib or abemaciclib were Grade 1 (visual effects that do not interfere with activities of daily living and asymptomatic heart rate decrease), except in three patients who experienced Grade 2 visual effects that resolved without dose reduction (one patient treated with camizestrant plus palbociclib and two patients treated with camizestrant plus abemaciclib) [46–48]. Ophthalmological review of patients reporting visual effects yielded no evidence of physical abnormalities [48].
Design
Study design
SERENA-6 (NCT04964934) is a ctDNA-guided phase III, randomized, double-blind study that examines the efficacy and safety of switching the endocrine therapy partner of CDK4/6i therapy from an AI to camizestrant when ESR1m is detected in ctDNA, but before clinical or radiological disease progression as per investigator assessment. The study has two steps, as summarized in Figure 2.
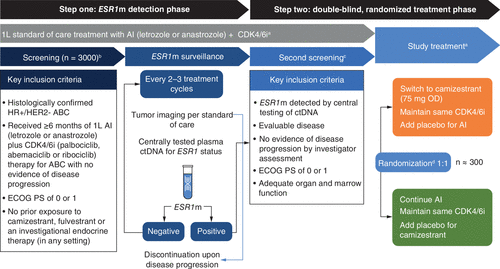
aPremenopausal/perimenopausal women or male participants (if medically indicated) receive a concurrent monthly luteinizing-hormone-releasing hormone agonist (goserelin or leuprorelin).
bPatients who are screen failures for STEP 1 can be rescreened.
cPatients who are screen failures for STEP 2 can be rescreened after consultation with the Global Study Team.
dRandomization will be stratified by: disease site (visceral disease vs non-visceral disease); ESR1m status (detectable at first versus subsequent ctDNA tests); time from initiation of CDK4/6i + AI to randomization (<18 months vs ≥18 months); CDK4/6i.
1L: First-line; ABC: Advanced breast cancer; AI: Aromatase inhibitor; CDK4/6i: Inhibitor of cyclin-dependent kinase 4/6; ctDNA: Circulating tumor DNA; ECOG: Eastern Cooperative Oncology Group; ESR1m: Estrogen receptor 1 gene mutation; HER2-: Human epidermal growth factor receptor 2-negative; HR+: Hormone receptor-positive; OD: Once daily; PS: Performance status.
In STEP ONE, patients who had received an AI plus CDK4/6i for at least 6 months are screened for the first set of inclusion and exclusion criteria (Table 2). Those who are eligible are tested for the presence of ESR1m in ctDNA every 2–3 treatment cycles (8–12 weeks) while continuing to receive SOC first-line therapy (an AI plus a CDK4/6i). In STEP ONE, continuous ctDNA monitoring will occur until disease progression is detected. At this point, the patient will either discontinue standard first-line treatment and exit the study, or ESR1m is detected, at which point patients will enter STEP TWO. Patients meeting additional eligibility criteria (Table 3) who have not progressed on staging scans are randomized double-blind 1:1 either to continue the same SOC therapy in STEP ONE augmented with camizestrant placebo or to receive camizestrant (75 mg once daily) and AI placebo with the same CDK4/6i at the same dose received in STEP ONE. The treatment phase of STEP TWO continues until disease progression, death or withdrawal for any reason.
| Inclusion criteria |
|---|
| Men and women ≥18 years of age (≥20 years in Japan) |
| Diagnosis of locoregionally recurrent or metastatic adenocarcinoma of the breast not amenable to resection or radiation therapy with curative intent |
| Histologically confirmed HR+/HER2- status based on local laboratory results |
| Receiving an AI (letrozole or anastrozole) plus a CDK4/6i (palbociclib, abemaciclib or ribociclib) for ≥6 months without clinical or radiological evidence of disease progression as per investigator assessment |
| May have received one previous line of chemotherapy for their locoregionally recurrent or metastatic breast cancer before CDK4/6i plus AI |
| Eastern Cooperative Oncology Group performance status of 0 or 1 |
| Minimum life expectancy of ≥6 months |
| Exclusion criteria |
| Previous exposure to camizestrant, fulvestrant or any investigational endocrine therapy (in any setting) |
| Currently treated with endocrine therapy (AI, tamoxifen or fulvestrant) alone for advanced disease irrespective of treatment duration |
| Risk of life-threatening complications in the short term |
| Uncontrolled or symptomatic CNS metastases, carcinomatous meningitis or leptomeningeal disease |
| No evidence of disease or bone-only disease with sclerotic/osteoblastic bone lesions |
| Severe or uncontrolled systemic disease |
| Chronic gastrointestinal disease, inability to swallow the formulated product, or previous significant bowel resection that would preclude adequate absorption, distribution, metabolism or excretion of CDK4/6i and AI |
| Currently pregnant (confirmed with a positive pregnancy test) or breastfeeding |
| Participation in a study of an investigational agent or device ≤4 weeks before the first dose of study treatment |
Eligibility criteria
Key eligibility criteria to enter STEP ONE of SERENA-6 are shown in Table 2. Briefly, patients must have histologically confirmed HR+/HER2- ABC and be currently receiving first-line AI (letrozole or anastrozole) plus a CDK4/6i (palbociclib, abemaciclib or ribociclib) in the advanced setting for 6 months or more without evidence of disease progression. Patients may have received one previous line of chemotherapy but must not have received other endocrine therapies for their locoregionally recurrent or metastatic ER+/HER2- ABC before CDK4/6i and AI treatment.
The additional eligibility criteria required to progress to STEP TWO are shown in Table 3. In addition to the detection of ESR1m in ctDNA, patients must have evaluable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and show no clinical or radiological evidence of disease progression.
| Inclusion criteria |
|---|
| Qualifying ESR1m detected in ctDNA |
| At least one evaluable lesion (not previously irradiated) that can be accurately assessed at baseline by CT or MRI and is suitable for repeated assessment. Measurable disease not required |
| Patients with bone disease only must have at least one non-previously irradiated lytic or mixed (lytic + sclerotic) bone lesion |
| Adequate organ and marrow function |
| Exclusion criteria |
| Clinical or radiological evidence of disease progression by investigator assessment and per RECIST v1.1 criteria |
| Concurrent use of non-topical hormonal therapy for non-cancer-related conditions (e.g., hormone replacement therapy) |
| Active infection, including uncontrolled tuberculosis, HBV, HCV or HIV |
Planned sample size
In total, SERENA-6 is expected to open at approximately 220 sites across 25 countries and will enroll approximately 3000 patients into STEP ONE. It is expected that approximately 300 patients will be randomized in STEP TWO to receive study treatment.
Study procedures
Blood samples will be collected from eligible consenting patients on entry to STEP ONE. ctDNA will be analyzed using the Guardant360 CDx assay (Guardant Health, CA, USA). An ESR1m test will be considered positive if one or more of the 11 most prevalent ESR1 mutations that occur in HR+ mBC [54] are detected (all of which cause constitutive activation of ER in preclinical models [40]. Patients without detectable ESR1m will continue receiving SOC first-line therapy. Blood samples for serial ctDNA testing will be taken every 8–12 weeks, coinciding with routine clinical visits conducted in concordance with patient management guidelines for HR+/HER2- ABC. If a ctDNA test is positive for ESR1m, the patient will progress to STEP TWO. Patients who experience disease progression without ESR1m during the surveillance period will be consented (optional) to perform comprehensive mutational landscape assessment.
At STEP TWO, patient consent will be requested for further eligibility testing and entry to the treatment phase of SERENA-6 (Figure 2 & Table 3). Patients who enter the treatment phase will be randomized 1:1 to receive camizestrant plus CDK4/6i or continue the AI plus CDK4/6i therapy received during STEP ONE. Randomization will be stratified by disease site (visceral disease versus non-visceral disease), ESR1m status (detectable at first versus subsequent ctDNA tests), time from initiation of CDK4/6i + AI to randomization (<18 months versus ≥18 months), and CDK4/6i. After randomization, the patient, physician and sponsor will be blinded to study treatment allocation. Unblinding of the physician may occur at a patient level to determine the best treatment during the trial. An independent data monitoring committee will review unblinded safety data and interim efficacy data and make recommendations on continuing the study based on their analysis.
Patients in the camizestrant group will receive camizestrant 75 mg (oral, once daily) plus CDK4/6i (continuing at the same dose and schedule as in STEP ONE), plus AI placebo. Patients in the AI group will receive an AI (continuing anastrozole [1 mg once daily] or letrozole [2.5 mg once daily]) plus CDK4/6i (maintained at the same dose and schedule as in STEP ONE), plus camizestrant placebo. All premenopausal or perimenopausal female or male patients (as medically applicable) will continue to receive a luteinizing hormone-releasing hormone agonist (goserelin or leuprorelin). The treatment phase of STEP TWO will be continued until RECIST v1.1-defined objective radiological progression as assessed by the investigator, unacceptable toxicity, withdrawal of consent, or death. In the event of toxicity that is not attributable to a disease-associated process, the dose of camizestrant/placebo may be reduced on one occasion (per patient) to 50 mg once daily. Temporary discontinuations of study treatment of up to 3 weeks are allowed for the management of some toxicities. Patients who discontinue study treatment prior to objective RECIST v1.1-defined radiological progression will be followed up with tumor assessments until RECIST v1.1-defined second disease progression or death. All patients who discontinue study treatment will be followed up for safety assessments as soon as possible after their last dose of study treatment, with additional assessments performed at the 28-day safety follow-up visit. Following disease progression, patients may continue to receive study treatment (camizestrant plus CDK4/6i or AI plus CDK4/6i) if the investigator judges that it continues to provide clinical benefit after discussion with the patient and sponsor. Otherwise, physicians will select a subsequent therapy according to the most recent US National Cancer Center Network (NCCN), ESMO, Pan-Asian adapted ESMO, or other internationally recognized guidelines, considering tumor mutation status.
End points
The primary end point is PFS, defined as the time from randomization until progression as per RECIST v1.1, as assessed by the investigator at the local site, or death due to any cause. Secondary end points include time to second progression event (PFS2), defined as the time from randomization to the earliest of the progression events (following the initial progression) after the first subsequent therapy or death. PFS2 provides longer-term information on patient survival than standard PFS, and its use is encouraged by some clinical trial guidelines [55]. Other secondary end points include chemotherapy-free survival, OS, safety and patient-reported outcomes (Table 4). Exploratory end points include analyzing ctDNA for predictive markers of response and/or acquired resistance to camizestrant as well as other potential molecular mechanisms of resistance to endocrine and CDK4/6i therapy.
| End point | Definition |
|---|---|
| PFS2 | Time from randomization to the earliest progression event (following the initial progression) after the first subsequent therapy or death |
| Chemotherapy-free survival | Time from randomization to the earliest start date of chemotherapy or death |
| Overall survival | Time from randomization until the date of death due to any cause |
| Safety and tolerability | Safety and tolerability will be evaluated in terms of type, incidence, severity (as graded by NCI-CTCAE v5.0), seriousness and relationship to study treatments of AEs, vital signs, clinical laboratory results, ECG recordings, echocardiogram recordings, and ophthalmological assessments |
| Health-related quality of life for patient-reported outcomes | As evaluated by EORTC QLQ-C30 and EORTC QLQ-BR23 |
Statistical analyses
Populations for analysis will include the full analysis set, which will be all intention-to-treat randomized patients. The safety analysis set includes all patients who have received at least one dose of study treatment.
PFS, PFS2 and OS will be analyzed using a log-rank test adjusted for stratification factors. A stratified Cox proportional hazards model will be used to estimate the HR and associated 95% CIs for each end point, and Kaplan–Meier plots will be presented by treatment group. The study is powered for both the primary end point, PFS, and the key secondary end point (PFS2).
Descriptive statistics will examine AEs by type, incidence, severity (as graded by US National Cancer Institute Common Terminology Criteria for Adverse Events [NCI-CTCAE] v5.0), seriousness, and relationship to study treatments. Vital signs, clinical laboratory results, cardiac and ophthalmological assessment values will also be examined.
Ethical considerations
The protocol, any protocol amendments, and all other relevant documents will be approved by an institutional review board or independent ethics committee at each participating institution before the study is initiated. SERENA-6 will be conducted in accordance with consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable Good Clinical Practice Guidelines, and all applicable local laws and regulations. Written, informed consent will be obtained from all patients before enrollment to STEP ONE and STEP TWO of the study. This manuscript was prepared in accordance with the SPIRIT reporting guidelines [56].
Conclusion
SERENA-6 examines the efficacy and safety of switching the endocrine therapy partner of first-line CDK4/6i therapy from an AI to camizestrant at the earliest time point when ESR1m is detected in ctDNA from patients with HR+/HER2- ABC but before clinical disease progression. SERENA-6 enrollment began in June 2021. Efficacy will be measured by end points including PFS, PFS2 and OS. SERENA-6 is the first study in HR+/HER2- ABC to utilize real-time prospective liquid biopsy monitoring to detect a molecular marker of treatment resistance and to switch patients to a next-generation SERD that targets the resistance mechanism at the time of detection. The rationale underlying SERENA-6 also raises the question of whether patients should receive a next-generation SERD rather than an AI from the outset of initial diagnosis of metastatic breast cancer, regardless of ESR1m status. This question is being addressed by SERENA-4 (NCT04711252), an ongoing phase III randomized, double-blind study that is evaluating the efficacy and safety of camizestrant plus palbociclib versus anastrozole plus palbociclib as first-line therapy for patients with HR+/HER2- ABC who have not received systemic treatment for advanced disease [57].
SERENA-6 study rationale
Current guidelines recommend combining endocrine therapy with a cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) as first-line treatment for patients with hormone-receptor positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC).
Almost all HR+ tumors become resistant to endocrine-based therapy, with ESR1m associated with acquired resistance to aromatase inhibitor (AI) and more aggressive disease features.
The PADA-1 trial demonstrated that progression-free survival (PFS) is prolonged if patients are switched from an AI to a first-generation selective estrogen receptor degrader (SERD) (fulvestrant) when estrogen receptor 1 gene mutation (ESR1m) becomes detectable in circulating tumor DNA (ctDNA).
Camizestrant is a potent next-generation oral SERD and pure estrogen receptor (ER) antagonist. Preclinical data demonstrated improved antitumor activity compared with fulvestrant in patient-derived xenograft models with ESR1m, including models with Y537S, which is putatively associated with resistance to fulvestrant. In a phase I study, camizestrant demonstrated promising clinical activity as monotherapy or in combination with a CDK4/6i in patients with ER+/HER2- ABC, including in patients whose tumors carried ESR1m. In a phase II study, camizestrant demonstrated a statistically significant PFS benefit versus fulvestrant in post-menopausal patients with ER+ ABC previously treated with endocrine therapy. The PFS benefit extended to subgroups with detectable ESR1m at baseline.
The recommended phase III dose of camizestrant at 75 mg daily has shown a tolerable safety profile, including when combined with a CDK4/6i.
SERENA-6 study design
SERENA-6 is a phase III, parallel, randomized, double-blind, matched placebo, multicenter, international study assessing the efficacy and safety of switching patients with HR+/HER2- ABC that has acquired ESR1m and not clinically progressed to camizestrant plus CDK4/6i versus continuing AI plus CDK4/6i.
Eligible patients are currently receiving an AI plus a CDK4/6i (palbociclib, abemaciclib or ribociclib) as first-line therapy for at least 6 months without disease progression. Patients with previous exposure to a SERD (in any setting) are not eligible.
ctDNA will be serially screened for ESR1m every 2–3 treatment cycles while patients continue the original AI plus CDK4/6i therapy.
Patients with detectable ESR1m, but no overt disease progression, will be randomized 1:1 to continue the original AI plus CDK4/6i therapy or to switch to camizestrant 75 mg (oral, once daily) plus CDK4/6i therapy.
The primary end point is PFS, according to RECIST v1.1, as assessed by local investigators. Secondary end points include PFS2, chemotherapy-free survival, overall survival (OS), safety and patient-reported outcomes.
Enrollment began in June 2021 and is expected at approximately 220 sites across 25 countries.
Supplementary data
An infographic and a supplementary podcast accompany this paper. To view or download these in your browser please click here: https://www.futuremedicine.com/doi/suppl/10.2217/fon-2022-1196
Author contributions
All authors met the criteria for authorship set forth by the International Committee of Medical Journal Editors and were involved in the conception, preparation, and approval of the manuscript.
Financial & competing interests disclosure
SERENA-6 is sponsored by AstraZeneca. G Bianchini reports consulting fees/consultant honorarium from Roche, AstraZeneca, Daiichi Sankyo, Eli Lilly, MSD, Chugai, Eisai, Gilead, Seagen, Sanofi, Exact Science and Agendia. F-C Bidard reports consulting fees/consultant honorarium from AstraZeneca, Institut Curie, Menarini Stemline, Eli Lilly and Sanofi; and holds intellectual property with Institut Curie. S Chia reports consulting fees/consultant honorarium from AstraZeneca, Novartis, Eli Lilly and Merck. M Cristofanilli reports grants/contracts to his institution from Celcuity, AstraZeneca, Daiichi Sankyo, Incyte, and Orum; consultant honorarium from Menarini, Ellipses, Eli Lilly, Pfizer; honorarium for speaking bureau from Pfizer; other honoraria from Guardant Health, and Foundation Medicine; and participation on an advisory board steering committee for AstraZeneca. S Fox reports employment from AstraZeneca. C Huang-Bartlett reports stock options/employment from AstraZeneca and Pfizer. H Iwata reports consulting fees/consultant honorarium from Chugai, Eli Lilly, AstraZeneca, Daiichi Sankyo, Pfizer, Kyowa Hakko Kirin Co, Taiho, MSD, Sanofi, Bayer, Boehringer Ingelheim, Novartis and Eisai. W Janni reports consulting fees/consultant honorarium from AstraZeneca, Celgene, Chugai, Daiichi Sankyo, Eisai, Exact Science, GSK, Janssen, Eli Lilly, Menarini, MSD, Novartis, Sanofi-Aventis, Roche, Pfizer and Seagen. K Kalinsky reports grants/contracts to his institution from Incyte, Novartis, Genentech, Eli Lilly and Company, Pfizer, Calithera Biosciences, Immunomedics, Acetylon Pharmaceuticals Inc., Seattle Genetics, Amgen, Zentalis Pharmaceuticals, and CytomX Therapeutics; consulting fees/consultant honorarium from Daiichi Sankyo, Eli Lilly, Pfizer, Novartis, Eisai, AstraZeneca, Immunomedics, Merck & Co, Seattle Genetics, Cyclocel, OncoSec Medical, and 4D Pharma plc; speakers bureau: Eli Lilly; support for attending meetings and/or travel from Eli Lilly, AstraZeneca, and Pfizer; participation on advisory board (steering committee): Immunomedics, AstraZeneca, Ambryx Biopharma, and Genentech; and stock options/employment: GRAIL (spouse), Array BioPharma (spouse), and Pfizer (spouse). X Liu reports stock options/employment from AstraZeneca; and stock options/employment: AstraZeneca (Spouse). CX Ma reports grants/contracts to her institution from Pfizer and Puma; consulting fees/ honorarium from Olaris and AstraZeneca; and participation on an advisory board for Novartis, Gilead, Sanofi-Genzyme, Biovica, Pfizer, Jacobio, Natera, Inivita, Athenex, Bayer and Eisai. EL Mayer reports consulting fees from AstraZeneca, Eli Lilly, Gilead and Novartis. S McClain reports employment from AstraZeneca. YH Park reports consulting fees/consultant honorarium from AstraZeneca, Pfizer, Hanmi, Roche, MSD, Daiichi Sankyo, Eli Lilly and Novartis; and holds intellectual property with Hanmi. N Turner has received advisory board honoraria from AstraZeneca, Bristol Myers Squibb, Eli Lilly, Merck Sharpe & Dohme, Novartis, Pfizer, Roche/Genentech, GlaxoSmithKline, Zentalis Pharmaceuticals, Repare Therapeutics, Arvinas, and research funding from AstraZeneca, BioRad, Pfizer, Roche/Genentech, Merck Sharpe & Dohme, Guardant Health, Invitae, Inivata, Personalis and Natera. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors thank R Goodchild from Oxford PharmaGenesis, and S Shaw from BOLDSCIENCE Inc. for writing assistance on this manuscript, which was funded by AstraZeneca.
Ethical conduct of research
The protocol, any protocol amendments, and all other relevant documents will be approved by an institutional review board or independent ethics committee at each participating institution before the study is initiated. SERENA-6 will be conducted in accordance with consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable GCP Guidelines, and all applicable local laws and regulations. Written, informed consent will be obtained from all patients before enrollment to STEP ONE and STEP TWO of the study.
Data-sharing statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data-sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. National Cancer Institute. Cancer Stat Facts: Female Breast Cancer Subtypes (2022). https://seer.cancer.gov/statfacts/html/breast-subtypes.html (Accessed 6 March 2023).
- 2. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 31(12), 1623–1649 (2020).
- 3. Latest generation estrogen receptor degraders for the treatment of hormone receptor-positive breast cancer. Expert Opin. Investig. Drugs 31(6), 515–529 (2022). • Summarizes information published to date on the efficacy, safety and pharmacokinetic profiles of new oral selective estrogen receptor degrader (SERD) drugs.
- 4. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J. Clin. Oncol. 39(35), 3959–3977 (2021).
- 5. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32(12), 1475–1495 (2021).
- 6. . Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 395(10226), 817–827 (2020).
- 7. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 21(2), 250–260 (2020).
- 8. The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer 27(3), 322–331 (2020).
- 9. Real-world treatment patterns and clinical outcomes in patients receiving palbociclib combinations for HR+/HER2- advanced/metastatic breast cancer in Japan: results from the IRIS study. Cancer Treat. Res. Commun. 32, 100573 (2022).
- 10. Real-world treatment of patients with palbociclib for HR+/HER2-advanced/metastatic breast cancer: the Europe IRIS study. Future Oncol. 18(3), 349–362 (2022).
- 11. . Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: results from the IRIS study. Breast 43, 22–27 (2019).
- 12. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J. Clin. Oncol. 40(28), 3246–3256 (2022). •• The first report of phase III data on an oral selective estrogen receptor (ER) modulator (SERM)/SERD, including showing that progression-free survival (PFS) of estrogen receptor 1 gene mutation (ESR1m)-positive breast cancer is longer with elacestrant than fulvestrant.
- 13. Results from VERONICA: a randomized, phase II study of second-/third-line venetoclax (VEN) + fulvestrant (F) versus F alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (LA/MBC). J. Clin. Oncol. 39(Suppl. 15), 1004–1004 (2021).
- 14. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 45(12), 1439–1445 (2013).
- 15. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345(6193), 216–220 (2014).
- 16. Prognostic impact of ESR1 mutations in ER+ HER2- MBC patients prior treated with first line AI and palbociclib: an exploratory analysis of the PADA-1 trial. J. Clin. Oncol. 38(Suppl. 15), 1010 (2020).
- 17. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 7(313), 313ra182 (2015).
- 18. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin. Cancer Res. 26(19), 5172–5177 (2020). • A report on the frequency of ESR1m in a large cohort of patients who had received prior AI therapy, and the association between the presence of ESR1m and reduced efficacy of aromatase inhibitor (AI) and fulvestrant.
- 19. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2(10), 1310–1315 (2016). • This secondary analysis of circulating tumor DNA (ctDNA) samples from >500 patients previously treated with an AI reports the frequency of ESR1m D538G and Y537S and their association with reduced survival.
- 20. Acquired genomic alterations in circulating tumor DNA from patients receiving abemaciclib alone or in combination with endocrine therapy. J. Clin. Oncol. 38(Suppl. 15), 3519–3519 (2020).
- 21. Real-world clinical-genomic data identifies the ESR1 clonal and subclonal circulating tumor DNA (ctDNA) landscape and provides insight into clinical outcomes. Cancer Res. 81(Suppl. 4), PS18-15 (2021).
- 22. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial-PEARL. Ann. Oncol. 32(4), 488–499 (2021).
- 23. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat. Commun. 9(1), 896 (2018).
- 24. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1):, a randomised, open-label, multicentre, phase III trial. Lancet Oncol. 23(11), 1367–1377 (2022). •• Report of the PADA-1 trial. The results show the PFS advantage of switching the endocrine partner of palbociclib from AI to fulvestrant upon detection of ESR1m in ctDNA.
- 25. Emergence of ESR1 mutation in cell-free DNA during first line aromatase inhibitor and palbociclib: an exploratory analysis of the PADA-1 trial. Ann. Oncol. 30, v105–v106 (2019).
- 26. . Early switch to fulvestrant plus palbociclib improves outcomes in ESR1-mutated, estrogen receptor-positive metastatic breast cancer. The Oncologist 27(Suppl. 1), 9–10 (2022).
- 27. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann. Oncol. 29(1), 145–153 (2018).
- 28. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res. 22(1), 56 (2020).
- 29. Impact of ESR1 mutations on endocrine therapy (ET) plus alpelisib benefit in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-), PIK3CA-mutated, advanced breast cancer (ABC) who progressed on or after prior cyclin-dependent kinase inhibitor (CDK4/6i) therapy in the BYLieve trial. Cancer Res. 82(Suppl. 4), PD15-01 (2022).
- 30. Allele-specific chromatin recruitment and therapeutic vulnerabilities of ESR1 activating mutations. Cancer Cell 33(2), 173–186; e175 (2018).
- 31. Understanding the organ tropism of metastatic breast cancer through the combination of liquid biopsy tools. Eur. J. Cancer 143, 147–157 (2021).
- 32. . Clinical implications of ESR1 mutations in hormone receptor-positive advanced breast cancer. Front Oncol. 7, 26 (2017).
- 33. . ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 23(1), 85 (2021).
- 34. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 21(10), 1296–1308 (2020).
- 35. Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin. Cancer Res. 28(16), 3433–3442 (2022). •• Exploratory subgroup analyses on data from the PALOMA-3 trial of patients with disease that progressed on prior endocrine therapy associate the presence of ESR1m with shorter PFS on fulvestrant and worse overall survival.
- 36. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann. Oncol. 33(8), 750–768 (2022).
- 37. . Oral selective estrogen receptor degraders (SERDs) in breast cancer: advances, challenges, and current status. Drug Des. Devel. Ther. 16, 2933–2948 (2022). • Summarizes information published to date on the efficacy, safety and pharmacokinetic profiles of new oral SERD drugs.
- 38. Alpelisib (ALP)+fulvestrant (FUL) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): biomarker (BM) analyses by next-generation sequencing (NGS) from the SOLAR-1 study. J. Clin. Oncol. 40(Suppl. 16), 1006 (2022).
- 39. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patients: study design of PADA-1. BMJ Open 12(3), e055821 (2022).
- 40. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov. 7(3), 277–287 (2017).
- 41. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: present and future from a clinical perspective. Int. J. Mol. Sci. 22(15), 7812 (2021).
- 42. . Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr. Relat. Cancer 22(5), 713–724 (2015).
- 43. Sanofi. Sanofi provides update on amcenestrant clinical development program (2022). https://ml-eu.globenewswire.com/Resource/Download/47677065-8ef3-406c-b8db-284daf4d5997 (Accessed 17 August 2022).
- 44. AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nat. Commun. 13(1), 4116 (2022).
- 45. 211MO - Giredestrant (GD-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2- locally advances/metastatic breast cancer (LA/mBC): primary analysis of the phase II randomised, open-label acelERA BC study. Ann. Oncol. 33(Suppl. 7), S88–S121 (2022).
- 46. SERENA-1: updated analyses from a phase 1 study (parts C/D) of the next-generation oral SERD camizestrant (AZD9833) in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. J. Clin. Oncol. 40(Suppl. 16), 1032–1032 (2022).
- 47. SERENA-1: updated analyses from a phase 1 study (Parts G/H) of the next-generation oral selective estrogen receptor degrader (SERD) camizestrant (AZD9833) in combination with abemaciclib, in women with ER-positive, HER2-negative advanced breast cancer. Cancer Res. 83(Suppl. 5), P3-07-28 (2023).
- 48. Updated data from SERENA-1: A phase 1 dose escalation and expansion study of the next generation oral SERD AZD9833 as a monotherapy and in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. Cancer Res. 81(Suppl. 4), PS11-05 (2021).
- 49. Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: results of the randomized, multi-dose phase 2 SERENA-2 trial. Cancer Res. 83(Suppl. 5), GS3-02 (2023).
- 50. Discovery of AZD9833, a potent and orally bioavailable selective estrogen receptor degrader and antagonist. J. Med. Chem. 63(23), 14530–14559 (2020).
- 51. Preclinical pharmacokinetic and metabolic characterization of the next generation oral SERD AZD9833. Cancer Res. 80(Suppl. 16), 1042 (2020).
- 52. A phase I dose escalation and expansion study of the next generation oral SERD AZD9833 in women with ER-positive, HER2-negative advanced breast cancer. J. Clin. Oncol. 38(Suppl. 15), 1024 (2020).
- 53. Not all selective estrogen receptor degraders are equal – Preclinical comparison of AZD9833, AZD9496 and fulvestrant. Cancer Res. 80(Suppl. 16), 4379 (2020).
- 54. The genomic landscape of metastatic breast cancer: insights from 11,000 tumors. PLOS ONE 15(5), e0231999 (2020).
- 55. Systematic review and meta-analysis of correlation of progression-free survival-2 and overall survival in solid tumors. Front Oncol. 10, 1349 (2020).
- 56. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 346, e7586 (2013).
- 57. SERENA-4: A phase III comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive, HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. J. Clin. Oncol. 39(Suppl. 15), TPS1101 (2021).


































