The association between expression of prolactin receptor and lymph node involvement in triple-negative breast cancer
Abstract
Background: Triple-negative breast cancer (TNBC) is associated with a poor prognosis and requires more aggressive treatment. Aim: The study aimed to evaluate the prophetic role of the prolactin receptor (PRLR) in TNBC stratification. Materials & methods: In a retrospective study, 58 formalin-fixed paraffin-embedded tumor tissues from patients diagnosed with TNBC were examined for PRLR expression using immunohistochemistry. The potential associations between PRLR expression and tumor characteristics were assessed. Result: PRLR expression was negative in 36 (62%) patients and positive in 22 (38%) patients. The number of positive PRLR tumors was significantly higher in patients without lymph node involvement (p = 0.019). Conclusion: PRLR expression was negatively associated with lymph node invasion in TNBC.
Worldwide, breast cancer is the second leading cause of cancer death in women [1,2]. Triple-negative breast cancer (TNBC) is defined by a lack of expression of estrogen and progesterone receptors and human epidermal growth factor receptors [3]. This subtype of breast cancer is seen in approximately 10–20% of all people living with breast cancer and is associated with a poor prognosis and a higher risk of recurrence and death [3,4]. Studies on the biology of TNBC have revealed that it is a heterogeneous disease with diverse behavior [5].
The prognosis of breast cancer depends on several factors. The most important of these are the causes of smoking, obesity, lymph node involvement, estrogen and progesterone receptors, epidermal growth factor 2 receptors, protein p 53 human epidermal growth factor receptor-2 (HER-2) and prolactin receptor (PRLR). Lymph node involvement is a significant predictor. Patients without lymph node involvement have a 30% chance of recurrence, while in patients with lymph node involvement, there is a 75% chance of recurrence [6,7].
In groups of patients with different prognoses, many attempts have recently been made to use molecular profiles and sub classifications of TNBC patients to find patients who are candidates for more aggressive treatment approaches [8]. PRLR is one of the most controversial biomarkers in this field [9]. A better understanding of this heterogeneity may provide new opportunities for better stratification of patients, giving rise to a personalized, less aggressive treatment for those patients with superior prognostic criteria. Association of prolactin with several tumors is identified [10]. Prolactin is a hormone secreted mainly by the anterior pituitary gland and induces its signaling cascade through dimerization with PRLR [11]. While many investigations have highlighted the role of PRLR in promoting breast cancer tumorigenesis [12,13,14], other studies have suggested a potential suppressor role for PRLR in breast carcinogenesis [15,16]. In this respect, the expression of PRLR has been found to correlate with a favorable prognosis of breast cancer in some investigations [915,16,17,18. Moreover, activation of the PRL pathway has been attributed to the increased histologic differentiation of breast cancer [19]. A recent study has introduced PRL and its receptor as a sub classified predictor of pro-differentiation therapy in TNBC [20]. Accordingly, they suggested a potential new modality for TNBC stratification using the component of the PRL pathway. To shed more light on this proposed stratification role, we aimed to investigate how the expression of PRLR is associated with the tumor characteristics in TNBC.
Materials & methods
In a retrospective cohort study, formalin-fixed paraffin-embedded (FFPE) tumor tissues from patients diagnosed with TNBC who underwent surgical treatment at our center (Imam Hossein Hospital, Tehran, Iran) between 2007 and 2017 were collected. The clinical and demographic characteristics of the patients were extracted from their medical files. Patients with incomplete clinical or demographic characteristics were excluded from the study. In this respect, a total of 80 patients diagnosed with TNBC were identified during the assigned period, from which clinic demographic characteristics were inadequate in 22 patients. These patients were removed from the study. The final study was performed on the remaining 58 FFPE tumor tissues.
Subsequently, tissue microarray (TMA) blocks were constructed. To this aim, four different tumor regions were marked on H&E slides by the corresponding pathologist and were matched to the donor blocks. Then a microarray sample with a diameter of 0.6 mm was punctuated and arrayed into a new recipient paraffin block using a tissue arrayer machine. Four TMA blocks were constructed for each patient, each containing one different tumor region from the same FFPE tissue block. The TMA blocks then underwent immunohistochemistry (IHC) staining.
IHC was performed on a 4 μm tissue section using mouse monoclonal antibody antihuman PRLR. IHC staining in this market is cytoplasmic. The staining was performed using the envisioned method and according to the manufacturer’s instructions. In brief, TMA sections were mounted on poly-l-lysine coated slides and dried in an oven at 60°c for 60 min. After deparaffinization and rehydration, tissue sections were immersed in methanol containing 0.3% hydrogen peroxide for 20 min to block the potential endogenous peroxidase activity. Subsequently, the sections underwent antigen retrieval by autoclaving in citrate buffer (pH = 6) for 10 min. Then, the sections were incubated with primary antibody for one h at an optimal dilution of 500-times and secondary antibody (Envision System, Dako, Denmark) for 30 min. Afterward, the sections were treated 3.3′-diaminobenzidine (DAB, Dako) as the chromogen and counterstained with hematoxylin (Dako).
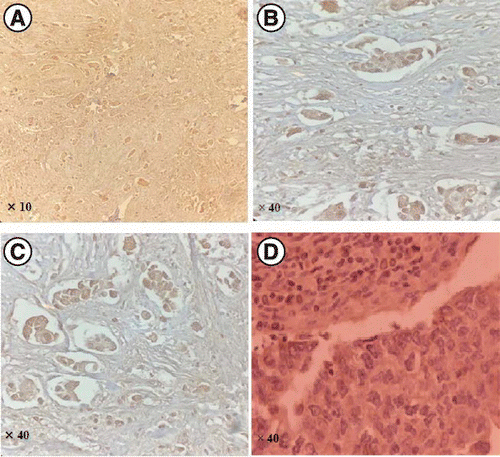
Finally, after the dehydration steps, the sections were mounted under glass coverslips and analyzed under a light microscope. The primary antibody was replaced with a washing buffer for negative control slides. The tumor was PRLR positive if more than 10% of the tumor cells were stained (Figure 1).
Statistical analysis
Statistical data analysis were performed using IBM SPSS for Windows, version 16. A descriptive analysis of the variables was done by the mean and standard deviation. The Chi-Square Test of Independence was used to assess potential associations between categorical variables. Analysis of variance or t-test evaluated potential associations between one numerical and one categorical variable. The Spearman correlation coefficient test assessed potential correlations. p-value of <0.05 was considered significant.
Results
A total of 58 formalin-fixed paraffin-embedded tumor tissues from 58 women diagnosed with TNBC were evaluated for PRLR expression using IHC. The mean age of the patients was 52 ± 10.7 years, ranging from 38 to 76 years. The mean tumor size was 4.6 ± 4.2 cm, ranging from 0.5 to 20 cm. Quadrantectomy was the most common type of treatment in patients. The clinical and demographic characteristics of the patients have been demonstrated in detail in Table 1.
| Variable | Mean ± SD or n (%) |
|---|---|
| Age (years) | 52 ± 10.7 |
| Type of surgery • Quadrantectomy • Radical mastectomy • Others | 43 (74.1%) 12 (20.7%) 3 (5.2%) |
| Menopause status • Positive • Negative | 51 (87.9%) 7 (12.1%) |
| Tumor size (cm) | 4.6 ± 4.3 |
| Lymph node • Positive • Negative | 24 (41.4) 34 (58.6) |
| Grade • 1 • 2 • 3 | 10 (17.3) 28 (48.3) 20 (34.4) |
| Stage • I • II • III • IV | 16 (27.6) 24 (41.4) 18 (31) 0 (0) |
PRLR expression was negative in 36 (62%) patients and positive in 22(38%) patients. No significant association was observed between PRLR expression and numerical variables, including age and tumor size (p = 0.17 and p = 0.7, respectively). Moreover, no significant association between PRLR expression and categorical variables, including the tumor stage and grade (p = 0.55 and p = 0.092, respectively). However, a significant association was observed between PRLR expression status and lymph node involvement. The number of PRLR-positive tumors was significantly higher in patients without lymph node involvement compared with patients with lymph node involvement (72.7 vs 27.3%; p = 0.019). While, no significant relationship was observed for other tumor characteristics such as tumor size, grade, and stage (p > 0.05) Table 2.
| Variable | PRLR | p-value | |
|---|---|---|---|
| Positive (n = 22) | Negative (n = 36) | ||
| Tumor size (cm) | 4.9 ± 3.91 | 4.4 ± 3.7 | 0.7 |
| Lymph node • Positive • Negative | 6 (27.3%) 16 (72.7%) | 18 (50%) 18 (50%) | 0.019 |
| Grade • 1 • 2 • 3 | 3 (13.7%) 10 (45.5%) 9 (40.8%) | 7 (19.4%) 18 (50%) 11 (30.6%) | 0.092 |
| Stage • I • II • III • IV | 5 (22.7%) 10 (45.5%) 7 (31.8%) 0 (0) | 11 (30.6%) 14 (38.8%) 11 (30.6%) 0 (0) | 0.55 |
Discussion
Human breast cancers represent a heterogeneous group of tumors with diverse behavior, outcomes and therapy response [21]. Nearly 1,671,149 new breast cancer cases were identified worldwide in 2012, from which 521,907 deaths (31.1%) due to breast cancer occurred [22]. To reduce the breast cancer mortality rate, developing prognostic markers capable of characterizing tumors of poor prognosis is of great value. These markers can ensure adequate therapy for each patient, giving rise to an improved patient outcome. Regarding TNBC as a more aggressive subgroup of breast cancer, more aggressive treatment has been implicated for these tumor types [21]. Even though emerging data demonstrate that even TNBC can be stratified into a range of aggressiveness [20], thus, the development of prognostic markers for TNBC stratification might allow a personalized, less aggressive treatment for those patients with superior prognostic criteria.
Several prognostic markers have been proposed to characterize TNBC to identify tumors with more aggressive behavior. In this respect, (Rakha et al., 2007) evaluated a large series of invasive breast carcinoma (n = 1944) with a long-term clinical follow-up (median, 56 months) to characterize a specific subgroup of breast cancer with more aggressive behavior [21]. According to their results, tumor size, lymph node stage and androgen receptor were the most useful prognostic markers. While both size and androgen receptor retained their prognostic significance in the lymph node-positive subgroup of their patients, the basal phenotype was the sole prognostic marker in the lymph node-negative tumors. Other prognostic markers, such as E-cadherin, P-cadherin and basal cytokeratins, have also been introduced [23,24,25]. Nevertheless, the search for a more specific prognostic marker for the stratification of TNBC continues.
Recently, PRLR has been widely discussed as a prognostic marker for breast cancer invasiveness. However, there is no clear consensus regarding the positive or negative impact of PRLR expression on the outcome of the affected patients.
Shemanko [26]; introduced PRLR as a marker for metastasis, specifically breast cancer bone metastasis. By contrast, the study of (Hachim et al., 2016) showed that PRLR expression was significantly associated with prolonged distant metastasis-free survival in breast cancer patients [15]. They suggested PRLR as an independent predictor of favorable prognosis in human breast cancer. López-Ozuna et al. [20], evaluated PRLR expression in a TNBC subtype of breast cancer. According to their results, gene expression of PRL signaling pathway components, including PRLR, could predict TNBC patients with significantly better survival outcomes [9]. To further clarify the role of PRLR as a prognostic marker of TNBC, we examined the potential associations between the PRLR expression and TNBC clinical characteristics in this study. According to our research, PRLR expression was negatively associated with lymph node invasion of TNBC. A more significant number of PRLR-positive tumors were lymph node-negative compared with PRLR-negative cases.
We did not find any other significant association between PRLR expression and tumor characteristics, such as tumor size, stage and grade. These results followed the study results of (López-Ozuna et al., 2016), showing PRLR may be a good prognosis marker in TNBC [20].
Our study has some weaknesses which should be pointed out. The small sample size could be regarded as the main limitation of this study. Other weaknesses of this study include the lack of overall survival or recurrence-free survival in patients with PRLR-positive breast cancer, BRCA mutation, and the lack of analysis of the pathological features of tumors due to lack of access to information from a large number of samples. Thus, further studies with a larger sample size are needed to clarify the role of PRLR expression in the prognosis of TNBC. Other weaknesses of this study include the pathologists examined the figure; however, it was not taken from an appropriate zone. Also, there was only a focal infiltration of stromal cells in the figure. Thus, we performed the staining again and provided relevant slides from different cases that confirmed only the tumor cells had been stained. The ideal method would be dual staining to differentiate the stromal and tumor cells. The consequence of this limitation is that tumor cells could not be differentiated from stromal cells in a few parts of the slides clearly.
Conclusion
According to our results, PRLR expression was negatively associated with lymph node invasion of TNBC. These results showed that PRLR may be a favorable prognosis marker in TNBC. However, further investigations with a larger sample size are warranted to shed more light on the role of PRLR in breast cancer pathogenesis, especially in the TNBC subtype of the tumor.
Prolactin receptor (PRLR) has been widely discussed as a prognostic marker for breast cancer invasiveness.
The associations between PRLR expression and tumor characteristics were assessed.
PRLR expression was negative in 62% of patients.
PRLR expression was negatively associated with lymph node invasion of triple-negative breast cancer.
There was no association between PRLR expression and tumor size.
There was no association between PRLR expression and tumor stage.
There was no association between PRLR expression and the age of patients.
PRLR may be a favorable prognosis marker in triple-negative breast cancer.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the Vice Chancellor at the Research and Ethics Committee for Research of Shahid Beheshti University of Medical Sciences, Tehran, Iran, with a number code (IR.SBMU.RETECH.REC.1396.741). This study’s research team adhered to the Helsinki Convention’s ethical principles regarding clinical studies in all stages of the present study. This study involved a retrospective review of medical records, and the ethics committee of Shahid Beheshti University of Medical Sciences waived the requirement for informed consent.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. . Overview of breast cancer. J. Am. Acad. PA 32(10), 13–17 (2019). •• This is the most updated information about breast cancer.
- 2. . Global trend of breast cancer mortality rate: a 25-year study. Asian Pac. J. Cancer Prev. 20(7), 2015–2020 (2019). •• This is the most updated global cancer statistics.
- 3. . The current state of breast cancer classification. Ann. Oncol. 23(Suppl. 10), x207–x210 (2012). •• The most up to date and best information for classification guidelines.
- 4. . Triple-negative breast cancer. N. Engl. J. Med. 363(20), 1938–1948 (2010).
- 5. . Subtyping of triple-negative breast cancer: implications for therapy. Cancer 121(1), 8–16 (2015). •• The most up to date and best information for subtyping of triple-negative breast cancer.
- 6. . Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109(9), 1721–1728 (2007). •• Provides comprehensive information about prolactin receptors (PRLR).
- 7. . Impact of number of positive lymph nodes and lymph node ratio on survival of women with node-positive breast cancer. Eur. J. Breast Health 15(2), 76–84 (2019).
- 8. . Prognostic and predictive biomarker developments in multiple myeloma. J. Hematol. Oncol. 14(1), 1–15 (2021).
- 9. Prolactin receptor expression as a novel prognostic biomarker for triple negative breast cancer patients. Ann. Diagn. Pathol. 46, 151507 (2020). •• Provides comprehensive information about PRLR expression.
- 10. . Steroid receptors in human breast cancer. Trend Endocrinol. Metab. 15(7), 316–323 (2004).
- 11. . Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19(3), 225–268 (1998).
- 12. . Prolactin cooperates with loss of p53 to promote claudin-low mammary carcinomas. Oncogene 33(23), 3075 (2014).
- 13. The role of prolactin in bone metastasis and breast cancer cell-mediated osteoclast differentiation. J. Natl Cancer Inst. 108(3), djv338 (2016).
- 14. . Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J. Clin. Investig. 100(11), 2744–2751 (1997).
- 15. . Prolactin receptor expression is an independent favorable prognostic marker in human breast cancer. Appl. Immunohistochem. Mol. Morphol. 24(4), 238–245 (2016). •• Provides comprehensive information about prognostic role of PRLR expression.
- 16. . Applied physiology of breast cancer. In: Breast Cancer. Sharma SCMazumdar AKaushik R (Eds). Springer, Singapore, 37–52 (2022).
- 17. . A favorable role of prolactin in human breast cancer reveals novel pathway-based gene signatures indicative of tumor differentiation and favorable patient outcome. Human Pathol. 53, 142–152 (2016).
- 18. Prolactin receptor-driven combined luminal and epithelial differentiation in breast cancer restricts plasticity, stemness, tumorigenesis and metastasis. Oncogenesis 10(1), 1–16 (2021).
- 19. Prolactin receptor expression and breast cancer: relationships with tumor characteristics among pre- and post-menopausal women in a population-based case-control study from Poland. Horm Cancer 5(1), 42–50 (2014).
- 20. . Prolactin pro-differentiation pathway in triple negative breast cancer: impact on prognosis and potential therapy. Sci. Rep. 6, 30934 (2016).
- 21. . Prognostic markers in triple-negative breast cancer. Cancer 109(1), 25–32 (2007).
- 22. . Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 17(S3), 43–46 (2016).
- 23. Prognostic factors in early-stage triple-negative breast cancer: lessons and limits from clinical practice. Anticancer Res. 33(6), 2737–2742 (2013).
- 24. Prognostic markers in triple-negative breast cancer (TNBC): the role of androgen receptor, e-cadherin, and Ki67. PLOS ONE. 10(7), e0132647 (2015).
- 25. P-cadherin expression as a prognostic biomarker in a 3992 case tissue microarray series of breast cancer. Modern Pathol. 24(1), 64 (2011).
- 26. . Prolactin receptor in breast cancer: marker for metastatic risk. J. Mol. Endocrinol. 57(4), R153–R165 (2016).

































