Patient characteristics associated with dose modifications for VRd among newly diagnosed multiple myeloma patients
Abstract
Aim: To evaluate among multiple myeloma (MM) patients, the proportions with first-line bortezomib/lenalidomide/dexamethasone (VRd) dose modifications and the associated baseline patient characteristics. Patients & methods: Adult MM patients treated with first-line VRd were selected from the Optum claims database. VRd dose modifications were defined based on lenalidomide dose. Results: Among 1497 MM patients, 33% received VRd lite and 22% VRd reduced. Compared with VRd regular, VRd lite usage was more likely to be associated with patients aged ≥75 years and female sex; VRd reduced usage was more likely to be associated with female sex and frailty. Conclusion: A large proportion of MM patients received VRd dose modifications in the real-world, which could potentially result in reduced effectiveness of VRd.
Tweetable abstract
In this real-world study of 1497 newly diagnosed multiple myeloma patients, over one-half received a modified dose, including 33% VRd lite and 22% VRd reduced. Usage of VRd lite was strongly associated with older age and usage of VRd reduced was associated with frailty.
Multiple myeloma (MM) is a hematological cancer that accounts for 1.8% of all new cancer cases with an estimated 34,470 new cases in 2022 in the USA [1]. It is primarily a cancer that affects older adults with a median age at diagnosis of 69 years [1]. Since this patient group may be affected by frailty and multiple comorbid conditions, including renal impairment, cardiovascular disease, osteoarthritis and others, their initial treatment can represent a challenge [2].
The most common regimens used in the USA for the treatment of transplant ineligible newly diagnosed multiple myeloma (NDMM) patients include lenalidomide, an immunomodulatory drug, in combination with dexamethasone (Rd), bortezomib, a proteasome inhibitor, in combination with lenalidomide and dexamethasone (VRd) and bortezomib in combination with dexamethasone (Vd), which together account for approximately two-thirds of the regimens used to treat patients with transplant ineligible NDMM [3,4]. VRd has become an established treatment for patients with sufficient fitness for triplet therapy, which is based on results from the open-label Southwest Oncology Group (SWOG S0777) clinical trial [5]. In this clinical trial of NDMM patients without intent for immediate transplant (VRd group: n = 242; median age: 63 years), standard dose VRd relative to Rd demonstrated superior outcomes, including (progression-free survival ([PFS] median: 43 months), overall survival median: 75 months and (overall response rate: 82%) [5]. The VRd regimen is also listed by the National Comprehensive Cancer Network (NCCN) guidelines as a preferred therapy for transplant ineligible NDMM patients and previously treated patients with MM [6].
Due to toxicities, such as neuropathy, thromboembolism, muscle weakness and infections [5], associated with the VRd regimen, and the older age and high prevalence of comorbid conditions among NDMM patients, dose modifications for VRd may be needed for some patients [2,5]. In prior clinical studies of MM patients treated with the Rd regimen, dose reductions were required, in particular for those who were elderly, because of toxicities [7–10]. In the phase II single-arm study of the dose modified VRd regimen, VRd lite (bortezomib = 1.3 mg/m2/lenalidomide = 15 mg/dexamethasone = 20 mg), it was shown to be efficacious in older MM patients (n = 50; median age: 73 years); median PFS was 35.1 months, median overall survival was not reached and overall response rate was 86% [11]. Among the patients in this clinical trial, peripheral neuropathy was experienced by 62% and two patients discontinued treatment because of toxicity [11]. This latter study prompts further analysis of patient outcomes associated with usage of dose modified VRd regimens among a larger and more generalizable patient population in the real-world setting. There is also limited real-world data on the demographic and clinical characteristics of patients with NDMM treated with dose modified VRd regimens. To address this knowledge gap, in this real-world study of MM patients treated with VRd first-line (1L) therapy, we evaluated the proportions of patients with dose modifications and the baseline patient characteristics associated with these dose modifications.
Patients & methods
Study design & data source
This was an observational, retrospective cohort study that used the Optum Clinformatics Data Mart® database, an adjudicated administrative healthcare claims database. The represented population in the Optum Clinformatics database is primarily US commercially insured patients, which also include those with Medicare Advantage insurance (≥65 years of age); however, ages are capped at 90 years. The administrative claims data includes inpatient and outpatient medical services and prescriptions as dispensed, as well as results for outpatient lab tests. Greater than 80 million patients with over 180 million claims are represented in this data source. Only deidentified patient data are available within the Optum Clinformatics database and it is fully compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Study population
Patients with ≥1 claim with a diagnosis code of MM (International Classification of Disease, 9th/10th Revision: ICD-9: 203.0×; ICD-10: C90.0×) between 1 July 2013 and 31 December 2020 were selected from the Optum claims database. From this MM patient group, those who had ≥1 medical or pharmacy claim for VRd as their first-line (1L) therapy between 1 November 2015 and 31 December 2020 were selected. The index date was defined as the first observed treatment initiation date of VRd; the MM diagnosis was required to be prior to the index date. Only patients with continuous health plan enrollment ≥6 months prior to the first observed MM diagnosis date (to reasonably ensure that patients were NDMM) were included in the study population. Continuous enrollment was also required between the MM diagnosis date and index date, with ≥1 day post index date. Patients were additionally required to be ≥18 years of age on their index date and have had no diagnoses of other malignancies or underwent a hematopoietic stem cell transplant from 6 months prior to the first observed MM diagnosis date to the index date. Patients were excluded if they had a diagnosis of amyloid light-chain amyloidosis during any time of the study period or if they had received any NCCN guideline recommended treatment prior to the index date. Patient attrition by eligibility criteria is shown in Figure 1.
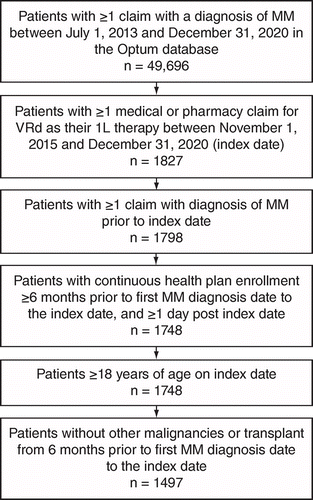
MM: Multiple myeloma; VRd: Bortezomib in combination with lenalidomide and dexamethasone.
Demographic & clinical characteristics
Demographics were evaluated on the index date and clinical characteristics were assessed during the baseline period (6 months prior to the index date) for patients in the overall study population and study cohorts grouped by the VRd dose regimen received (VRd regular, VRd lite, VRd reduced; see below for dose definitions). The evaluated demographic and clinical characteristics included age, sex, race, payer type (commercial or Medicare), health plan type, Quan Charlson Comorbidity Index (CCI) score [12], CRAB symptoms (hypercalcemia, renal impairment, anemia, bone disease) [13] and select comorbidities. Frailty was evaluated using the frailty index based on 31 health associated deficits (domains of morbidity, functional status, cognition, mood, sensory loss and other geriatric domains) [14]. Additionally, year of index date and time from first observed MM diagnosis to index date were measured for all patients.
VRd regimens & dose modifications
VRd regimen was identified using both medical and pharmacy claims. Patients were considered to have initiated VRd regimen if bortezomib and lenalidomide were received during the first 60 days following the index date. Patients were considered to have moved to the next line of therapy (LOT) on either the initiation date of a new regimen after discontinuation of VRd or the date of treatment change (including augmentation and switching), whichever occurred first. Changes in any agent within 60 days of index date was not considered a LOT change. The date of treatment change or new treatment was the initiation date of the next LOT regimen. A maximum gap of 90 days was allowed within a LOT. If an hematopoietic stem cell transplant occurred prior to the end of a gap, another 6-month allowed gap was added after the transplant.
The dose modifications of VRd were defined based on the lenalidomide dose received during 1L therapy. Receipt of >15 mg lenalidomide was categorized as VRd regular, ≤15 mg lenalidomide as VRd lite and initial lenalidomide dose >15 mg reduced to ≤15 mg over the 1L therapy as VRd reduced. The duration of 1L VRd therapy was measured as the time interval between the index date and the confirmed end date of the LOT.
Statistical analyses
Categorical variables were summarized with frequency and proportions and continuous variables with means and standard deviations.
Baseline patient characteristics associated with different VRd dose categories were evaluated using multinomial logistic regression models. The dependent variables in the models were the different doses of 1L VRd therapy (VRd lite, VRd reduced and VRd regular) with VRd regular as the reference group. The independent variables included age groups (<65, 65 to <70, 70 to <75, ≥75 years), sex, race, payer type, index date year, frailty index, Quan-CCI score and select comorbid conditions. Odds ratios (ORs) with 95% CIs were reported. All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., NC, USA).
Results
Demographic & clinical characteristics of study population
Among the 1497 MM patients included in the study population, mean age was 69 years (standard deviations: 9.5), 52% were ≥70 years of age, 51% were male, 59% were White and 67% had Medicare (Table 1). Over a third (37%) of patients were frail; mean baseline Quan CCI was 4.2 (Table 2). Forty percent of the patients had one or more CRAB symptoms, with 25% having renal impairment, 19% hypercalcemia, 18% anemia and 1% bone disease. The most prevalent comorbid conditions evaluated were hypertension (70%), hyperlipidemia (56%), diabetes (32%), osteoarthritis (30%) and cardiovascular conditions (25%).
| Overall n = 1497 | VRd regular n = 585 (39%) | VRd lite n = 498 (33%) | VRd reduced n = 327 (22%) | |
|---|---|---|---|---|
| Age at index, years, mean (SD) | 69 (9.5) | 66 (9) | 73 (9) | 68 (9) |
| Age group in years, n (%) | ||||
| <65 | 428 (29) | 240 (41) | 64 (13) | 105 (32) |
| 65 to <70 | 283 (19) | 131 (22) | 63 (13) | 62 (19) |
| 70 to <75 | 380 (25) | 133 (23) | 136 (27) | 88 (27) |
| ≥75 | 406 (27) | 81 (14) | 235 (47) | 72 (22) |
| Sex, n (%) | ||||
| Male | 769 (51) | 343 (59) | 222 (45) | 166 (51) |
| Female | 728 (49) | 242 (41) | 276 (55) | 161 (49) |
| Race, n (%) | ||||
| White | 882 (59) | 338 (58) | 287 (58) | 199 (61) |
| Black | 254 (17) | 96 (16) | 99 (20) | 46 (14) |
| Asian | 31 (2) | 13 (2) | 11 (2) | 7 (2) |
| Hispanic | 132 (9) | 58 (10) | 31 (6) | 33 (10) |
| Unknown | 198 (13) | 80 (14) | 70 (14) | 42 (13) |
| Payer type, n (%) | ||||
| Commercial | 500 (33) | 269 (46) | 79 (16) | 118 (36) |
| Medicare | 997 (67) | 316 (54) | 419 (84) | 209 (64) |
| Health plan type, n (%) | ||||
| Health maintenance organization | 216 (14) | 98 (17) | 72 (14) | 30 (9) |
| Preferred provider organization | 122 (8) | 40 (7) | 38 (8) | 35 (11) |
| Point of service | 359 (24) | 201 (34) | 46 (9) | 90 (28) |
| Exclusive provider organization | 52 (3) | 25 (4) | 14 (3) | 8 (2) |
| Indemnity | 19 (1) | 1 (0) | 11 (2) | 5 (2) |
| Others/multiple | 729 (49) | 220 (38) | 317 (64) | 159 (49) |
| Year of index date, n (%) | ||||
| 2015 | 29 (2) | 13 (2) | 12 (2) | 4 (1) |
| 2016 | 208 (14) | 85 (15) | 58 (12) | 47 (14) |
| 2017 | 258 (17) | 96 (16) | 91 (18) | 58 (18) |
| 2018 | 329 (22) | 131 (22) | 99 (20) | 77 (24) |
| 2019 | 359 (24) | 138 (24) | 118 (24) | 89 (27) |
| 2020 | 314 (21) | 122 (21) | 120 (24) | 52 (16) |
| Time from first observed MM Dx to index date, days, mean (SD) | 60 (186) | 63 (205) | 59 (173) | 55 (172) |
| Overall n = 1497 | VRd regular n = 585 | VRd lite n = 498 | VRd reduced n = 327 | |
|---|---|---|---|---|
| Frailty index >0.2, n (%) | 558 (37) | 162 (28) | 252 (51) | 111 (34) |
| Quan CCI, mean (SD) | 4.2 (2.3) | 4.2 (2.3) | 4.4 (2.2) | 4.0 (2.2) |
| CRAB symptoms, n (%) | ||||
| ≥1 symptom | 593 (40) | 202 (35) | 252 (51) | 103 (31) |
| Hypercalcemia | 290 (19) | 104 (18) | 111 (22) | 52 (16) |
| Renal impairment | 379 (25) | 110 (19) | 182 (37) | 61 (19) |
| Anemia | 271 (18) | 83 (14) | 135 (27) | 40 (12) |
| Bone disease | 10 (1) | 7 (1) | 1 (0.2) | 1 (0.3) |
| All | 0 | 0 | 0 | 0 |
| Comorbid conditions, n (%) | ||||
| Hypertension | 1048 (70) | 360 (62) | 419 (84) | 215 (66) |
| Hyperlipidemia | 835 (56) | 296 (51) | 307 (62) | 187 (57) |
| Diabetes | 483 (32) | 171 (35) | 195 (40) | 98 (20) |
| Osteoarthritis | 443 (30) | 156 (27) | 169 (34) | 96 (29) |
| Cardiovascular conditions† | 379 (25) | 131 (22) | 169 (34) | 61 (19) |
| Obesity | 323 (22) | 134 (23) | 107 (21) | 70 (21) |
| Peripheral neuropathy | 254 (17) | 94 (16) | 101 (20) | 47 (14) |
| Osteoporosis | 202 (13) | 70 (12) | 77 (15) | 42 (13) |
| Depression | 197 (13) | 72 (12) | 69 (14) | 41 (13) |
| Anxiety | 196 (13) | 72 (12) | 65 (13) | 48 (15) |
| COPD | 160 (11) | 59 (10) | 62 (12) | 33 (10) |
| Asthma | 120 (8) | 46 (8) | 43 (9) | 24 (7) |
| Peptic ulcer | 39 (3) | 13 (2) | 21 (4) | 5 (2) |
| Stroke/TIA | 36 (2) | 11 (2) | 21 (4) | 3 (1) |
| Rheumatoid arthritis | 26 (2) | 8 (1) | 9 (2) | 8 (2) |
VRd dose modifications
Over one-half of the patients received a modified VRd dose, including 33% VRd lite and 22% VRd reduced (Table 1); median duration of 1L VRd therapy was 4.9 months (among those with confirmed end of therapy: n = 1093; 73%).
Baseline patient characteristics associated with different VRd dose categories
Compared with VRd regular, usage of VRd lite was over six-times more likely to be associated with patients ≥75 years of age (OR: 6.16; 95% CI: 3.60–10.54) and over two-times more likely to be associated with patients 70 to <75 years of age (OR: 2.23; 95% CI: 1.31–3.78) compared with patients <65 years of age, and nearly two-times more likely to be associated with female sex (OR: 1.80; 95% CI: 1.36–2.37); it was approximately 40% less likely to be associated with commercial insurance versus Medicare (OR: 0.58; 95% CI: 0.37–0.93) (Table 3). Renal impairment (OR: 2.70; 95% CI: 1.75–4.17) and hypertension (OR: 1.90; 95% CI: 1.34–2.67) were also associated with usage of VRd lite versus VRd regular (Table 3). Other evaluated patient characteristics were not significantly different between VRd lite and VRd regular with the adjustment of other characteristics.
| VRd lite vs VRd regular OR (95% CI) | VRd reduced vs VRd regular OR (95% CI) | |
|---|---|---|
| Age at index (years) | ||
| 65 to <70 vs <65 | 1.03 (0.61–1.74) | 0.93 (0.58–1.51) |
| 70 to <75 vs <65 | 2.23 (1.31-3.78) | 1.22 (0.73–2.05) |
| ≥75 vs <65 | 6.16 (3.60-10.54) | 1.63 (0.94–2.83) |
| Sex | ||
| Female vs male | 1.80 (1.36-2.37) | 1.33 (1.01-1.77) |
| Race | ||
| Black vs White | 0.93 (0.64–1.35) | 0.77 (0.51–1.16) |
| Asian/other race vs White | 0.76 (0.55–1.07) | 0.90 (0.65–1.26) |
| Payer type | ||
| Commercial vs Medicare | 0.58 (0.37-0.93) | 0.82 (0.52–1.29) |
| Index date year: >2017 vs ≤2017 | 0.89 (0.66–1.19) | 0.94 (0.70–1.26) |
| Frailty index >0.2 vs ≤0.2 | 1.27 (0.90–1.78) | 1.53 (1.06-2.21) |
| Quan CCI score | 0.95 (0.88–1.01) | 0.97 (0.90–1.04) |
| Comorbid conditions | ||
| ≥1 CRAB symptom | 1.07 (0.73–1.58) | 0.77 (0.51–1.15) |
| Renal impairment | 2.70 (1.75-4.17) | 1.29 (0.80–2.08) |
| Diabetes | 1.13 (0.83–1.55) | 1.01 (0.73–1.40) |
| Peripheral neuropathy | 1.11 (0.77–1.58) | 0.84 (0.56–1.24) |
| Hypertension | 1.90 (1.34-2.67) | 1.10 (0.80–1.51) |
| Cardiovascular conditions† | 1.05 (0.75–1.46) | 0.66 (0.45-0.97) |
| Stroke/TIA | 1.32 (0.56–3.09) | 0.49 (0.13–1.84) |
Compared with VRd regular, usage of VRd reduced was 33% more likely to be associated with female sex (OR: 1.33; 95% CI: 1.01–1.77) and 53% more likely to be associated with patients who were frail (OR: 1.53; 95% CI: 1.06–2.21); it was 34% less likely to be associated with patients who had cardiovascular conditions (OR: 0.66; 95% CI: 0.45–0.97) (Table 3). Other evaluated patient characteristics were not significantly different between VRd reduced and VRd regular with the adjustment of other characteristics.
Discussion
In this large real-world retrospective cohort study of MM patients who received 1L VRd therapy, a majority (52%) were over the age of 70 and over a third (37%) were considered frail according to a published algorithm for determination of frailty index [14]. Usage of modified dose VRd regimens was common, with over one-half of patients having received a modified dose, including 33% VRd lite and 22% VRd reduced. For comparison, in the open-label SWOG S0777 clinical trial (n = 264), only four patients over the age of 75 received a VRd dose reduction [5,11]. Thus, our study findings on the usage of modified dose VRd regimens among MM patients in routine clinical practice in the US highlight the differences in VRd therapy in the real-world versus clinical trial settings.
Among our study population in the real world, older age was strongly associated with receiving VRd lite, with patients aged 70–74 approximately two-times more likely to have received VRd lite versus VRd regular than those <65 years of age, and those 75 years of age and older more than six-times more likely to have received VRd lite. Other patient characteristics associated with the usage of VRd lite over VRd regular included female sex, renal impairment and hypertension. Patient characteristics associated with receiving a reduction in VRd lenalidomide dose during 1L therapy were female sex and frailty. These findings emphasize the need for follow-up studies to examine the impact of these frequently used dose modifications of the VRd regimen on the outcomes of MM patients in the real-world setting, particularly among those subgroups that have been identified in this study as more likely to receive such modified VRd regimens.
As noted earlier, in our real-world study, 52% of the patients were 70 years of age and older. With the aging of the US population, not only is the number of patients annually diagnosed with MM projected to increase but also the predominance of older aged MM patients [15]. Although survival has improved among the MM patient population with recent treatment advances, older age remains predictive of worse outcomes [16,17]. Therefore, it is especially important that older aged patients with NDMM receive optimized effective treatments [18,19]. Frailty (37%), diabetes (32%), osteoarthritis (30%), renal impairment (25%) and cardiovascular conditions (25%) were highly prevalent in our study population. Such comorbid conditions, alongside older age, considerably complicate treatment management, which has to balance effectiveness with toxicity and tolerability for individual patients. Moreover, these patient characteristics influence the choice of treatment as well as patient response to treatment (e.g., therapy discontinuation and treatment related mortality) [16,20]. Other treatments that do not require frequent dose modifications may provide improved clinical benefits and need to be considered in older patient groups with frailty and multiple comorbidities.
Although the findings from the phase II study of VRd lite indicate that when using the modified dose among older transplant ineligible MM patients, VRd efficacy is maintained with manageable toxicity, the sample size was very small (n = 50 patients) [11]. The findings of our current study show the frequency of VRd lite usage is high in real-world settings, especially among older and more vulnerable patients with NDMM. In a recently conducted real-world study of 2342 NDMM patients treated with 1L VRd therapy, it was found that median PFS was shorter among those who were elderly (≥75 years of age), frail, and those who had renal impairment compared with the overall study population [21]. Thus, it is additionally important to determine whether a relationship exists between VRd dose regimen and PFS among patient subgroups in real-world settings. A goal of such scientific research would be to assist in defining the best treatment, drug combinations and doses for particular patient subgroups, especially the older or frail populations, in efforts to extend disease remission and improve outcomes among all MM patients.
Limitations
This study was a claims database analysis, and the study findings should be interpreted in this context. First, the MM patient population in this study represents those with US commercial insurance and/or employer-sponsored Medicare coverage and thus, while the study findings are generalizable to such MM patients across the US, they may not be generalizable to MM patients with different insurance types or no insurance or those in other countries. Second, similar to other claims data, the Optum Clinformatics database consists of claims submitted by healthcare providers for reimbursement and these claims may contain possible coding errors, coding for the purpose of rule-out and under-/upper-coding. Third, regarding pharmacy medications, the data only reflect those filled by patients and may not capture all which were actually taken by patients. Fourth, a 6-month period prior to the first observed MM diagnosis date in which no MM diagnosis was observed (i.e., wash-out period) was utilized and such a timeframe may not have been sufficient to ensure all patients were newly diagnosed with MM. However, considering the average duration from first observed MM diagnosis date to patients’ index dates (i.e., initiation of VRd regimen) was 60 days and a patient was required to have not received any NCCN guideline recommended treatment prior to their index date, there is a high likelihood that most patients captured were NDMM. Fifth, 1L VRd therapy was determined from medical and pharmacy claims and a patient was considered to have received a 1L VRd regimen if bortezomib and lenalidomide were received within the first 60 days following the index date, which may not always reflect the prescribing practice of physicians. Sixth, in the real-world setting, dose modifications to the VRd regimen may also include modifying the dosage of bortezomib and further study using data sources that provide bortezomib dosage information is warranted. Lastly, due to limitations of the database and incomplete data, we were not able to include other patient disease factors, such as results from specific laboratory tests and genetic screening, in the evaluation of patient characteristics associated with usage of modified VRd regimens.
Conclusion
To summarize, in this real-world retrospective cohort study of 1497 MM patients treated with 1L VRd therapy in the USA, a large proportion (52%) were over the age of 70 and over a third (37%) were frail. VRd dose modifications were common, with over one-half of the study population having received a modified dose of VRd, including 33% VRd lite and 22% VRd reduced. Usage of VRd lite was strongly associated with older age, while usage of VRd reduced was associated with frailty. These commonly used dose modifications of the VRd regimen could potentially result in reduced effectiveness of VRd in the real-world relative to controlled clinical trials, which needs to be assessed in future studies, especially among more vulnerable patients with NDMM as defined herein. Gaining a better understanding of the best treatments, drug combinations and doses for particular patient subgroups in routine oncology practice will be helpful toward providing all MM patients with the best clinical benefits of treatment.
Due to toxicities associated with the VRd regimen and the older age and high prevalence of comorbid conditions among newly diagnosed multiple myeloma (NDMM) patients, dose modifications for VRd may be needed for some patients.
There is limited real-world data on the demographic and clinical characteristics, in addition to outcomes, of patients with NDMM treated with dose modified VRd regimens.
In this study of 1497 patients with NDMM treated with 1L VRd in the real world, we found that over one-half received a modified dose of VRd, including 33% VRd lite and 22% VRd reduced.
Reduction of VRd dose was more common in more vulnerable patient populations, including older patients, frail patients and those with renal impairment.
Such dose modifications of the VRd regimen could potentially result in reduced effectiveness of VRd in the real-world relative to controlled clinical trials, which needs to be assessed in future studies.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
T Ran, R Medhekar, AZ Fu, S Patel and S Kaila contributed to study concept, design and data interpretation. T Ran and AZ Fu contributed to statistical analyses. T Ran, R Medhekar, AZ Fu, S Patel and S Kaila contributed to drafting and revising of the manuscript.
Financial & competing interests disclosure
T Ran, R Medhekar, AZ Fu, S Patel and S Kaila are employees of Janssen Scientific Affairs, LLC. This work was supported by Janssen Scientific Affairs, LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and editorial support for preparation of this manuscript was provided by J Lin and M Lingohr-Smith of Novosys Health and was funded by Janssen Scientific Affairs, LLC.
Ethical conduct of research
The data source used in this study (i.e., the Optum Clinformatics Data Mart® database) contains deidentified patient data sets and is fully compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Papers of special note have been highlighted as: • of interest; •• of considerable interest
References
- 1. SEER Cancer Stat Facts: Myeloma. National Cancer Institute, MD, USA, https://seer.cancer.gov/statfacts/html/mulmy.html
- 2. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 118(17), 4519–4529 (2011).
- 3. . Treatment patterns and clinical and economic outcomes inpatients with newly diagnosed multiple myeloma treated with lenalidomide- and/or bortezomib containing regimens without stem cell transplant in a real-world setting. Clin. Lymphoma Myeloma Leuk. 19(10), 645–655 (2019).
- 4. Treatment pattern and outcomes in newly diagnosed multiple myeloma patients who did not receive autologous stem cell transplantation: a real-world observational study. Adv. Ther. 38(1), 640–659 (2021).
- 5. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase III trial. Lancet 389(10068), 519–527 (2017). •• The SWOG S0777 trial shows that VRd, a triplet regimen, compared to Rd, a doublet regimen, improved response rates, progression-free survival and overall survival.
- 6. Multiple Myeloma, Version 5.2022, NCCN Clinical Practice Guidelines in Oncology. www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf •• NCCN guidelines for the treatment of multiple myeloma (MM) patients.
- 7. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 23(11), 2147–2152 (2009).
- 8. Upfront lower dose lenalidomide is less toxic and does not compromise efficacy for vulnerable patients with relapsed refractory multiple myeloma: final analysis of the phase II RevLite study. Br. J. Haematol. 177(3), 441–448 (2017).
- 9. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 357(21), 2123–2132 (2007).
- 10. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N. Engl. J. Med. 357(21), 2133–2142 (2007).
- 11. A Phase II study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br. J. Hematol. 182(2), 222–230 (2018). • A clinical trial that shows VRd lite is efficacious for the treatment of transplant ineligible MM patients, although the study population was small (n = 50 patients).
- 12. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 173(6), 676–682 (2011).
- 13. . Development of an algorithm to distinguish smoldering versus symptomatic multiple myeloma in claims-based data sets. JCO Clin. Cancer Inform. 1, CCI.17.00089 (2017).
- 14. . Frailty in older adults with multiple myeloma: a study of US Veterans. JCO Clin. Cancer Inform. 4, 117–127 (2020). • Utilizes a claims-based algorithm to assess frailty.
- 15. . Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood 125(2), 410–412 (2015).
- 16. . Age disparities in survival from lymphoma and myeloma: a comparison between US and England. Br. J. Haematol. 165(6), 824–831 (2014).
- 17. . Management of newly diagnosed elderly multiple myeloma patients. Curr. Oncol. Rep. 21(7), 64 (2019).
- 18. . Management of multiple myeloma in older adults: gaining ground with geriatric assessment. J. Geriatr. Oncol. 8(1), 1–7 (2017).
- 19. . Geriatric assessments and frailty scores in multiple myeloma patients: a needed tool for individualized treatment? Curr. Opin. Oncol. 33(6), 648–657 (2021).
- 20. . Survival trends in elderly myeloma patients. Eur. J. Haematol. 106(1), 126–131 (2021).
- 21. . Real-world patient characteristics and treatment outcomes among nontransplanted multiple myeloma patients who received bortezomib in combination with lenalidomide and dexamethasone as first line of therapy in the United States. BMC Cancer 22(1), 901 (2022). • This retrospective observational cohort study examines outcomes of MM patients treated with VRd as 1L therapy and found that its real-world effectiveness was less than reported in the clinical trial setting.

































